AI Breakthrough: Designing Synthetic DNA Switches for Precise Gene Control
6 Sources
6 Sources
[1]
AI Helps Scientists Decode DNA Switches for Targeted Gene Control
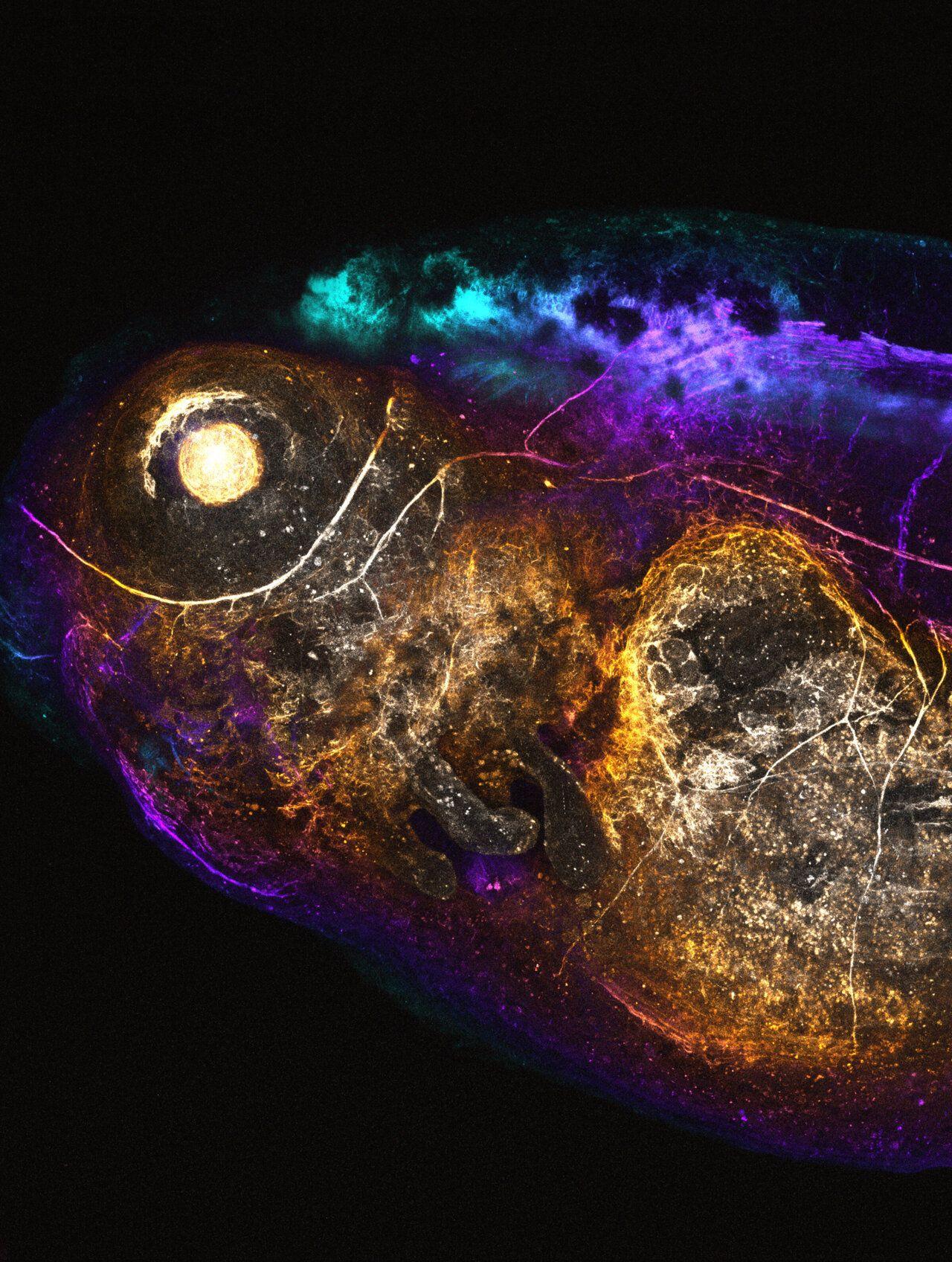
AI reveals "DNA grammar" for precise gene editing Synthetic DNA switches enable targeted gene activity New method supports selective cell-specific gene therapy Researchers have used AI to understand patterns in DNA sequences that control when and where genes are active. This work, from The Jackson Laboratory, MIT's Broad Institute, and Yale University, introduces a new tool for precise gene editing. The focus of this study was on DNA regions called cis-regulatory elements (CREs). These are like on-off switches that ensure specific genes are only active in the correct cells. While CREs help direct genes to work in one cell type and not others, the rules -- or "grammar" -- for how CREs work has been tough to figure out. To decode these rules, the team analyzed large sets of DNA data using an AI model. This model identified CRE patterns that help activate or suppress genes in particular cells. Researchers then used these insights to design synthetic DNA switches, aimed at specific tissues. The team tested these synthetic switches, or CREs, in animal models to see how well they worked. They observed successful results, like activating a fluorescent marker only in the liver cells of zebrafish embryos without affecting other tissues. This precise targeting shows the potential for future therapies that would activate genes in just one tissue or organ. Using this AI approach, scientists created thousands of new CREs, each with unique functions for targeted gene control. This development may lead to genetic treatments for conditions that need specific cell targeting. This tool may advance gene editing for research, but it also opens doors for targeted treatments. With the ability to turn genes on or off in precise cell types, scientists see applications in treating genetic conditions or improving tissue repair. As lead researcher Ryan Tewhey noted, this approach could let researchers "fine-tune gene activity in one tissue," paving the way for treatments with minimal side effects on other cells.
[2]
AI-designed DNA switches flip genes on and off, allowing precise activation or repression
Researchers at The Jackson Laboratory (JAX), the Broad Institute of MIT and Harvard, and Yale University, have used artificial intelligence to design thousands of new DNA switches that can precisely control the expression of a gene in different cell types. Their new approach opens the possibility of controlling when and where genes are expressed in the body, for the benefit of human health and medical research, in ways never before possible. "What is special about these synthetically designed elements is that they show remarkable specificity to the target cell type they were designed for," said Ryan Tewhey, Ph.D., an associate professor at The Jackson Laboratory and co-senior author of the work. "This creates the opportunity for us to turn the expression of a gene up or down in just one tissue without affecting the rest of the body." In recent years, genetic editing technologies and other gene therapy approaches have given scientists the ability to alter the genes inside living cells. However, affecting genes only in selected cell types or tissues, rather than across an entire organism, has been difficult. That is in part because of the ongoing challenge of understanding the DNA switches, called cis-regulatory elements (CREs), that control the expression and repression of genes. In a paper published in Oct. 23 online issue of Nature, Tewhey and his collaborators not only designed new, never-before-seen synthetic CREs, but used the CREs to successfully activate genes in brain, liver or blood cells without turning on those genes in other cell types. Tissue- and time-specific instructions Although every cell in an organism contains the same genes, not all the genes are needed in every cell, or at all times. CREs help ensure that genes needed in the brain are not used by skin cells, for instance, or that genes required during early development are not activated in adults. CREs themselves are not part of genes, but are separate, regulatory DNA sequences -- often located near the genes they control. Scientists know that there are thousands of different CREs in the human genome, each with slightly different roles. But the grammar of CREs has been hard to figure out, "with no straightforward rules that control what each CRE does," explained Rodrigo Castro, Ph.D., a computational scientist in the Tewhey lab at JAX and co-first author of the new paper. "This limits our ability to design gene therapies that only affect certain cell types in the human body." "This project essentially asks the question: 'Can we learn to read and write the code of these regulatory elements?'" said Steven Reilly, Ph.D., assistant professor of genetics at Yale and one of the senior authors of the study. "If we think about it in terms of language, the grammar and syntax of these elements is poorly understood. And so, we tried to build machine learning methods that could learn a more complex code than we could do on our own." Using a form of artificial intelligence (AI) called deep learning, the group trained a model using hundreds of thousands of DNA sequences from the human genome that they measured in the laboratory for CRE activity in three types of cells: blood, liver and brain. The AI model allowed the researchers to predict the activity of any sequence from the almost infinite number of possible combinations. By analyzing these predictions, the researchers discovered new patterns in the DNA, learning how the grammar of CRE sequences in the DNA impacts how much RNA would be made -- a proxy for how much a gene is activated. The team, including Pardis Sabeti, MD, DPhil, co-senior author of the study and a core institute member at the Broad Institute and professor at Harvard, then developed a platform called CODA (Computational Optimization of DNA Activity), which used their AI model to efficiently design thousands of completely new CREs with requested characteristics, like activating a particular gene in human liver cells but not activating the same gene in human blood or brain cells. Through an iterative combination of 'wet' and 'dry' investigation, using experimental data to first build and then validate computational models, the researchers refined and improved the program's ability to predict the biological impact of each CRE and enabled the design of specific CREs never before seen in nature. "Natural CREs, while plentiful, represent a tiny fraction of possible genetic elements and are constrained in their function by natural selection," said study co-first author Sager Gosai, Ph.D., a postdoctoral fellow in Sabeti's lab. "These AI tools have immense potential for designing genetic switches that precisely tune gene expression for novel applications, such as biomanufacturing and therapeutics, that lie outside the scope of evolutionary pressures." Pick-and-choose your organ Tewhey and his colleagues tested the new, AI-designed synthetic CREs by adding them into cells and measuring how well they activated genes in the desired cell type, as well as how good they were at avoiding gene expression in other cells. The new CREs, they discovered, were even more cell-type-specific than naturally occurring CREs known to be associated with the cell types. "The synthetic CREs semantically diverged so far from natural elements that predictions for their effectiveness seemed implausible," said Gosai. "We initially expected many of the sequences would misbehave inside living cells." "It was a thrilling surprise to us just how good CODA was at designing these elements," said Castro. Tewhey and his collaborators studied why the synthetic CREs were able to outperform naturally occurring CREs and discovered that the cell-specific synthetic CREs contained combinations of sequences responsible for expressing genes in the target cell types, as well as sequences that repressed or turned off the gene in the other cell types. Finally, the group tested several of the synthetic CRE sequences in zebrafish and mice, with good results. One CRE, for instance, was able to activate a fluorescent protein in developing zebrafish livers but not in any other areas of the fish. "This technology paves the way toward the writing of new regulatory elements with pre-defined functions," said Tewhey. "Such tools will be valuable for basic research but also could have significant biomedical implications where you could use these elements to control gene expression in very specific cell types for therapeutic purposes."
[3]
Researchers flip genes on and off with AI-designed DNA switches
Researchers at The Jackson Laboratory (JAX), the Broad Institute of MIT and Harvard, and Yale University, have used artificial intelligence to design thousands of new DNA switches that can precisely control the expression of a gene in different cell types. Their new approach opens the possibility of controlling when and where genes are expressed in the body, for the benefit of human health and medical research, in ways never before possible. "What is special about these synthetically designed elements is that they show remarkable specificity to the target cell type they were designed for," said Ryan Tewhey, PhD, an associate professor at The Jackson Laboratory and co-senior author of the work. "This creates the opportunity for us to turn the expression of a gene up or down in just one tissue without affecting the rest of the body." In recent years, genetic editing technologies and other gene therapy approaches have given scientists the ability to alter the genes inside living cells. However, affecting genes only in selected cell types or tissues, rather than across an entire organism, has been difficult. That is in part because of the ongoing challenge of understanding the DNA switches, called cis-regulatory elements (CREs), that control the expression and repression of genes. In a paper published in Oct. 23 advanced online issue of Nature, Tewhey and his collaborators not only designed new, never-before-seen synthetic CREs, but used the CREs to successfully activate genes in brain, liver or blood cells without turning on those genes in other cell types. Tissue- and time-specific instructions Although every cell in an organism contains the same genes, not all the genes are needed in every cell, or at all times. CREs help ensure that genes needed in the brain are not used by skin cells, for instance, or that genes required during early development are not activated in adults. CREs themselves are not part of genes, but are separate, regulatory DNA sequences -- often located near the genes they control. Scientists know that there are thousands of different CREs in the human genome, each with slightly different roles. But the grammar of CREs has been hard to figure out, "with no straightforward rules that control what each CRE does," explained Rodrigo Castro, PhD, a computational scientist in the Tewhey lab at JAX and co-first author of the new paper. "This limits our ability to design gene therapies that only effect certain cell types in the human body." "This project essentially asks the question: 'Can we learn to read and write the code of these regulatory elements?'" said Steven Reilly, PhD, assistant professor of genetics at Yale and one of the senior authors of the study. "If we think about it in terms of language, the grammar and syntax of these elements is poorly understood. And so, we tried to build machine learning methods that could learn a more complex code than we could do on our own." Using a form of artificial intelligence (AI) called deep learning, the group trained a model using hundreds of thousands of DNA sequences from the human genome that they measured in the laboratory for CRE activity in three types of cells: blood, liver and brain. The AI model allowed the researchers to predict the activity for any sequence from the almost infinite number of possible combinations. By analyzing these predictions, the researchers discovered new patterns in the DNA, learning how the grammar of CRE sequences in the DNA impact how much RNA would be made -- a proxy for how much a gene is activated. The team, including Pardis Sabeti, MD, DPhil, co-senior author of the study and a core institute member at the Broad Institute and professor at Harvard, then developed a platform called CODA (Computational Optimization of DNA Activity), which used their AI model to efficiently design thousands of completely new CREs with requested characteristics, like activating a particular gene in human liver cells but not activating the same gene in human blood or brain cells. Through an iterative combination of 'wet' and 'dry' investigation, using experimental data to first build and then validate computational models, the researchers refined and improved the program's ability to predict the biological impact of each CRE and enabled the design of specific CREs never before seen in nature. "Natural CREs, while plentiful, represent a tiny fraction of possible genetic elements and are constrained in their function by natural selection," said study co-first author Sager Gosai, PhD, a postdoctoral fellow in Sabeti's lab. "These AI tools have immense potential for designing genetic switches that precisely tune gene expression for novel applications, such as biomanufacturing and therapeutics, that lie outside the scope of evolutionary pressures." Pick-and-choose your organ Tewhey and his colleagues tested the new, AI-designed synthetic CREs by adding them into cells and measuring how well they activated genes in the desired cell type, as well as how good they were at avoiding gene expression in other cells. The new CREs, they discovered, were even more cell-type-specific than naturally occurring CREs known to be associated with the cell types. "The synthetic CREs semantically diverged so far from natural elements that predictions for their effectiveness seemed implausible," said Gosai. "We initially expected many of the sequences would misbehave inside living cells." "It was a thrilling surprise to us just how good CODA was at designing these elements," said Castro. Tewhey and his collaborators studied why the synthetic CREs were able to outperform naturally occurring CREs and discovered that the cell-specific synthetic CREs contained combinations of sequences responsible for expressing genes in the target cell types, as well as sequences that repressed or turned off the gene in the other cell types. Finally, the group tested several of the synthetic CRE sequences in zebrafish and mice, with good results. One CRE, for instance, was able to activate a fluorescent protein in developing zebrafish livers but not in any other areas of the fish. "This technology paves the way toward the writing of new regulatory elements with pre-defined functions," said Tewhey. "Such tools will be valuable for basic research but also could have significant biomedical implications where you could use these elements to control gene expression in very specific cell types for therapeutic purposes."
[4]
AI helps flip DNA switches; paving way for precision treatment
By analyzing vast amounts of DNA data, the AI model was able to uncover patterns that humans couldn't easily identify. Researchers at The Jackson Laboratory (JAX), the Broad Institute of MIT and Harvard, and Yale University have achieved a breakthrough in gene control through artificial intelligence (AI). The novel approach uses AI to design synthetic DNA switches, known as cis-regulatory elements (CREs), which can precisely regulate gene activity in specific tissues or cell types. A key challenge in genetic engineering has been the ability to control where and when genes are expressed in living organisms. While gene-editing technologies like CRISPR have made it easier to modify genes, ensuring that these alterations only impact the desired tissues or cells has been difficult.
[5]
Generative AI Designs DNA to Switch Genes On and O | Newswise
Newswise -- A team of researchers from Yale School of Medicine (YSM), the Jackson Laboratory, and the Broad Institute of M.I.T. and Harvard has developed a new AI tool capable of designing never-before-seen sequences of synthetic DNA capable of switching on targeted genes in specific cells. The breakthrough could pave the way for improved gene therapies. Researchers describe the new AI platform, called Computational Optimization of DNA Activity (CODA), in an article published October 23rd in Nature. The authors say while gene therapies hold the potential to rewrite disease-causing mutations, better methods are needed to deliver the therapy directly to cells that harbor disease, while leaving it inactive in other parts of the body where it could cause harm. The CODA tool creates DNA sequences, called cis-regulatory elements (CREs), which use a complex code to activate genes in specific cells. "This project essentially asks the question: 'Can we learn to read and write the code of these regulatory elements?'" said Steven Reilly, PhD, assistant professor of genetics at YSM and one of the senior authors of the study. "If we think about it in terms of language, the grammar and syntax of these elements is poorly understood. And so, we tried to build machine learning methods that could learn a more complex code than we could do on our own." The team trained its model on over 775,000 naturally-occurring CREs tested in blood, liver, and brain-related cell types, then tested the novel, AI-generated CREs in the same type of cells. Researchers say in many cases, the synthetic CREs were more specific to a given cell type than natural ones. "There are a lot of potential solutions out there for lots of different possible things you might want a regulatory element to do," Reilly said. "Evolution maybe has never wanted to build a really great driver for an Alzheimer's drug, but that doesn't mean it can't exist." The paper's first authors were Rodrigo Castro, PhD, of the Jackson Laboratory and Sager Gosai, PhD, of the Broad Institute. The senior authors were Steven Reilly, PhD, of Yale School of Medicine, Pardis Sabeti, PhD, of the Broad Institute, and Ryan Tewhey, PhD, of the Jackson Laboratory.
[6]
AI-designed DNA sequences regulate cell-type-specific gene expression
Different parts of a cell's genome can be active or inactive depending on the cell's function in the body, and whether it is in a disease state. The instructions for activating or repressing a gene are encoded in the genome, and each type of cell has its own genomic 'language' that is based on highly complex patterns of nucleotides that describe whether a gene will be expressed. Writing in Nature, Gosai et al. apply artificial intelligence (AI) methods to learn the 'regulatory grammar' of that language -- that is, the patterns of nucleotides in the genome that relate to gene-regulatory activity -- for different cell types. The authors then apply those models, along with experimental genomics techniques, to create synthetic DNA sequences that can drive gene expression in specific cell types, which has implications for targeted cell and gene therapy. The ability to create synthetic sequences that can target specific cell populations has arisen from advances in both experimental genomics and AI. In the past 15 years, the field of molecular biology has seen the development of massively parallel reporter assays (MPRAs), which measure the activity of hundreds of thousands of 'regulatory elements' in the genome. These short sequences are positioned between genes and often contain binding sites for transcription factors. Whether a regulatory element is active (that is, has activating transcription factors bound) depends on the cell type, and so these sequences drive the expression of genes in a cell-type-dependent manner. Using MPRAs, it is possible to identify which regulatory elements are active in a given cell type, effectively enabling specific cell types to be 'targeted'. In a preprint earlier this year, researchers in the same laboratory as Gosai and colleagues describe their application of this technology to assess how hundreds of thousands of mutations that are associated with human diseases affect different cell types. In parallel, the AI techniques that have revolutionized image analysis over the past decade have also improved scientists' ability to learn the language of the genome across different types of cell. Networks of artificial neurons (known as convolutional neural networks, or CNNs) can learn features of an image that enable them to distinguish images of cats from images of dogs, for example. In the same way, CNNs can learn the important genome-sequence features of regulatory elements that enable cell types to be distinguished -- such as the combination of transcription-factor binding sites that lead to gene expression in one type of cell but not in another. In the current study, Gosai and colleagues integrate these emerging computational and experimental technologies: measurements made by MPRAs serve as input to train the genomic AI models. After training, these computational models generate synthetic, regulatory-element sequences that emulate the cell-type specificity of the natural sequences that the models were trained on (Fig. 1). MPRAs also provide the means to test which of these synthesized sequences are the most specific to certain cell types. Applying this approach, the researchers show that their synthetic sequences drive gene expression in a manner that is more specific to the target cells than are any of the regulatory elements found in nature. Gosai and colleagues' study is a part of a wider trend towards accelerating biological discoveries by tightly integrating AI and experimental biology. A pair of studies published online in December 2023 reported the development of AI methods to create hundreds of synthetic sequences, the function of which could be tested in fruit flies. The integration of AI and experimental biology is increasingly being used in other domains as well -- for example, to create CRISPR proteins for genome editing that have fewer off-target effects than any such proteins found in nature. These and other findings mark a transition in the field of genomics from describing biology to engineering it. The ability to create highly specific synthetic sequences has exciting applications for cell and gene therapies, in which disease-causing cells or genes (such as those harbouring mutations) are replaced with functioning ones. One of the main challenges in designing a gene therapy is avoiding off-target effects. Replacing a dysfunctional protein in one cell type can lead to an effective treatment, but the same protein can lead to toxic side effects if it is delivered to a different type of cell. Therefore, synthetic sequences that are active in specific cell populations have the potential to limit the toxicity of a wide variety of gene therapies. There are still fundamental obstacles to overcome before this technology can reach the clinic. The ability to measure the function of thousands of genome sequences is currently limited to fruit flies and to cultured cells that are grown in a dish. There might be features of those cells that do not translate well to humans. Alternative AI approaches to the one described by Gosai and colleagues rely on human data, but have so far been validated in only a handful of cell types, such as different classes of neuron. Bridging the gap between the lab and clinic in the case of synthetic sequence creation will probably involve the integration of new computational and experimental technologies. Generative AI methods, similar to those that underlie the chatbot ChatGPT, could facilitate the building of computational models that generalize across different cell types and species more effectively than current methods do. In terms of experimental work, an approach called spatial transcriptomics, in which gene-expression profiles can be mapped across tissue slices, could enable researchers to measure the specificity of hundreds of candidate synthetic regulatory elements in the context of a tissue. Beyond creating synthetic sequences, there is tremendous potential in further integrating emerging computational and experimental technologies. The next generation of biologists, computational biologists and biomedical engineers will need to be trained in ways that allow them to collaborate with researchers in other disciplines or, more excitingly, to integrate these highly complex fields themselves.
Share
Share
Copy Link
Researchers use AI to create synthetic DNA switches (CREs) that can precisely control gene expression in specific cell types, potentially revolutionizing gene therapy and targeted treatments.

AI Decodes DNA "Grammar" for Precise Gene Control
Researchers from The Jackson Laboratory, MIT's Broad Institute, and Yale University have made a significant breakthrough in gene control using artificial intelligence (AI). The team has developed an AI model capable of designing synthetic DNA switches, known as cis-regulatory elements (CREs), that can precisely regulate gene activity in specific tissues or cell types
1
2
.Understanding CREs and the Challenge of Gene Control
CREs are DNA sequences that act as on-off switches, ensuring genes are only active in the correct cells. While every cell in an organism contains the same genes, not all genes are needed in every cell or at all times. CREs help ensure that genes needed in one cell type, such as the brain, are not activated in others, like skin cells
3
.The challenge has been understanding the complex "grammar" of these CREs, which has limited scientists' ability to design gene therapies that only affect certain cell types in the human body. As Ryan Tewhey, Ph.D., an associate professor at The Jackson Laboratory and co-senior author of the study, explained, "This creates the opportunity for us to turn the expression of a gene up or down in just one tissue without affecting the rest of the body"
2
.AI-Powered Solution: CODA Platform
To tackle this challenge, the research team developed a platform called Computational Optimization of DNA Activity (CODA). This AI-driven tool uses deep learning to analyze hundreds of thousands of DNA sequences from the human genome, measuring CRE activity in three types of cells: blood, liver, and brain
3
4
.The AI model allowed researchers to:
- Predict the activity of any sequence from an almost infinite number of possible combinations
- Discover new patterns in DNA
- Learn how the grammar of CRE sequences impacts gene activation
- Design thousands of new, synthetic CREs with specific characteristics
Related Stories
Surprising Effectiveness of Synthetic CREs
When tested, the AI-designed synthetic CREs demonstrated remarkable specificity to their target cell types, often outperforming naturally occurring CREs. The researchers observed successful results, such as activating a fluorescent marker only in the liver cells of zebrafish embryos without affecting other tissues
1
5
.Sager Gosai, Ph.D., a postdoctoral fellow involved in the study, noted, "The synthetic CREs semantically diverged so far from natural elements that predictions for their effectiveness seemed implausible. We initially expected many of the sequences would misbehave inside living cells"
3
.Implications for Gene Therapy and Future Research
This breakthrough has significant implications for gene therapy and targeted treatments. The ability to control when and where genes are expressed in the body opens up new possibilities for addressing genetic conditions with minimal side effects on other cells
1
4
.Steven Reilly, Ph.D., assistant professor of genetics at Yale and one of the senior authors, highlighted the potential applications: "Evolution maybe has never wanted to build a really great driver for an Alzheimer's drug, but that doesn't mean it can't exist"
5
.As the field of AI-driven genetic engineering continues to evolve, this research paves the way for more precise and effective gene therapies, potentially revolutionizing the treatment of genetic disorders and advancing our understanding of gene regulation.
References
Summarized by
Navi
[2]
[3]
[4]
Related Stories
AI Designs DNA to Control Gene Expression in Healthy Mammalian Cells
09 May 2025•Science and Research

AI Model Predicts Gene Activity in Human Cells, Transforming Biological Research
09 Jan 2025•Science and Research

AI-Powered 'Pythia' Tool Revolutionizes Precision in CRISPR Gene Editing
13 Aug 2025•Science and Research
Recent Highlights
1
Google Gemini 3.1 Pro doubles reasoning score, beats rivals in key AI benchmarks
Technology

2
Pentagon Summons Anthropic CEO as $200M Contract Faces Supply Chain Risk Over AI Restrictions
Policy and Regulation

3
Canada Summons OpenAI Executives After ChatGPT User Became Mass Shooting Suspect
Policy and Regulation