AI-Designed Antibiotics Show Promise Against Drug-Resistant Superbugs
16 Sources
16 Sources
[1]
How Is AI Changing the Fight Against Drug-Resistant Bacteria?
The researchers synthesized a small subset of these AI-designed molecules and found them lethal to superbugs responsible for drug-resistant gonorrhea and stubborn staphylococcus skin infections. "It's a great addition to this emerging field of using AI for antibiotic discovery," says César de la Fuente, a synthetic biologist at the University of Pennsylvania who was not involved in the research. "It shows quite well how generative AI can produce molecules with real-world activity," he adds. "It's elegant and potentially clinically meaningful." A social-enterprise non-profit created by Collins, called Phare Bio, now plans to advance these and other AI-discovered antibiotics toward clinical development. The candidate antibiotics build on earlier finds from Collins' lab -- including halicin, a potent broad-spectrum antibiotic identified in 2020; a more targeted agent called abaucin with activity against Acinetobacter baumannii, a major cause of hospital-acquired infections; and a novel structural class of molecules described last year that proved effective against the superbugs MRSA and VRE. With the team's earlier discoveries, however, Collins and his colleagues were still mining existing chemical libraries, using deep-learning models to spot overlooked compounds with antibacterial potential. The new work sets down a new path altogether: rather than searching for hidden gems in familiar territory, the generative AI platform starts from scratch, conjuring entirely new molecular structures absent from any database. "This is moving from using AI as a discovery tool to using AI as a design tool," Collins says. The shift, he adds, opens new frontiers in antibiotic discovery -- unexplored territory that could harbor the next generation of lifesaving drugs. To train their generative AI model, Collins and his colleagues first used a neural network framework to virtually screen more than 45 million chemical fragments -- the building blocks of would-be drugs -- looking for pieces predicted to have activity against Neisseria gonorrhoeae (the cause of sexually transmitted gonorrhea infections) and Staphylococcus aureus (the germ behind deadly bloodstream infections, pneumonia, and flesh-eating skin disease). Two algorithms then went to work: one assembling the fragments into complete molecular structures, the other predicting which of those structures would pack the strongest antibacterial punch. Together, the algorithms generated more than 10 million candidate molecules, none of which had ever existed before. But then came what MIT study author and computational biologist Aarti Krishnan describes as "a massive bottleneck": very few of these prophesied antibiotics could actually be made in the lab. The researchers manually sifted through the AI hits, filtering for properties suggestive of drug-likeness and synthetic feasibility. They ultimately arrived at a shortlist of around 200 promising designs, 24 of which could be successfully generated. Seven proved to be bona fide antimicrobial agents, as confirmed by laboratory tests, with two showing particularly strong efficacy in mouse models of gonorrhea and staph infections. Notably, each seems to work through a distinct and novel mechanism of action not exploited by existing antibiotics. "That's pretty cool," says Phare co-founder Jonathan Stokes, an antimicrobial chemical biologist at Canada's McMaster University in Hamilton, Ontario. He praises Collins' team for unearthing two highly promising antibiotic leads but notes that the labor-intensive trial-and-error process underscores how far the technology still has to go in producing compounds that can be readily synthesized. "It's a bit of an elephant in the room," he says of synthetic tractability in GenAI drug discovery. "Antibiotics, because of the financial disincentives in this space, have to be cheap," Stokes, who was not involved in the research, says. "They have to be cheap to discover, cheap to develop, and cheap to make. So if there are opportunities to avoid all of these issues with synthetic feasibility, I feel like that is a major advantage." To tackle that challenge, Stokes and his colleagues developed a generative AI tool that designs antibiotic candidates with chemical blueprints tailored for real-world manufacturing, not just computer screens. This tool, called SyntheMol, operates within a more limited chemical space than Collins' GenAI model, choosing only molecules whose building blocks can be synthesized with known, lab-proven reaction steps. That narrows the search parameters to tens of billions of molecules, compared to the 10 possible structures that Collins' model explored. It's enough, however, for SyntheMol to have already yielded several drug candidates that Stokes and his colleagues, through a startup called Stoked Bio, hope to develop into treatments for bacteria linked to Crohn's disease and other hard-to-treat conditions. The team aims to balance the sheer breadth of biochemical possibilities the models can explore with crucial metrics like drug potency, safety, low toxicity, and ease of synthesis. "It's a multi-objective optimization problem," says de la Fuente, who advises Phare and builds his own generative AI models to design antimicrobial peptide drugs. But for now, the tools powering Phare's discovery efforts -- rooted in Collins' approaches -- are already delivering early wins, says Akhila Kosaraju, Phare Bio's CEO and president. "We are getting substantially more potent and less toxic initial compounds," she notes. And backed by the U.S. government's Advanced Research Projects Agency for Health (ARPA-H), along with the philanthropic arm of Google -- which is funding Phare to build open-source infrastructure around AI-guided antibiotic design -- Kosaraju and her colleagues aim to move the most promising candidates into human trials. "We are building what we think is the most novel and robust pipeline of antibiotics in the world," she says.
[2]
Generative AI Gave MIT Scientists a New Tool to Fight Antibiotic-Resistant Bacteria
Macy has been working for CNET for coming on 2 years. Prior to CNET, Macy received a North Carolina College Media Association award in sports writing. Antibiotic-resistant bacteria are dangerous because they already "know" what most antibiotics look like. Scientists at MIT have found a way to create something new: using generative AI to design two antibiotic compounds from scratch that can kill drug-resistant gonorrhea and MRSA in lab dishes and mice. Antibiotic resistance is one of the world's biggest public health threats yet new antibiotics have been scarce for decades. Traditional drug discovery methods rely on screening known chemical libraries -- a slow process with a limited pool of existing molecules to test. In contrast, MIT's AI system generated more than 36 million theoretical compounds, many with chemical structures never seen before, and zeroed in on two standouts. Both are unlike any antibiotic currently in use, offering a glimpse at how AI can move beyond speeding up research to imagine medicines that might have been impossible to find otherwise. "We wanted to get rid of anything that would look like an existing antibiotic, to help address the antimicrobial resistance crisis in a fundamentally different way," said Aarti Krishnan, MIT postdoc and one of the study's lead authors. "By venturing into underexplored areas of chemical space, our goal was to uncover novel mechanisms of action." Read also: Do You Really Learn When You Use AI? What MIT Researchers Found The MIT team bypassed the limitations of screening existing chemical libraries by asking AI to invent molecules from scratch, generating more than 36 million theoretical compounds, which were then narrowed down to a few to be tested against drug-resistant superbugs. This involved two AI-driven strategies: Fragment-based design: The AI began with a chemical fragment (labeled F1) that showed promise against gonorrhea. It produced millions of derivatives, ultimately refining a shortlist of about 1,000 candidates. Of the 80 chosen by researchers, NG1 emerged as a standout compound that successfully treated drug-resistant gonorrhea in cell cultures and a mouse. Unconstrained generation: The team let the AI roam freely, designing molecules on its own, aiming at MRSA. This produced more than 29 million candidates, which were filtered down to 90 compounds for synthesis. Twenty-two were produced, six performed well in lab tests and one in particular, DN1, proved able to eliminate MRSA skin infections in mice. NG1 and DN1 are structurally distinct from any currently known antibiotics and appear to destroy bacteria by disrupting their cell membranes. NG1 specifically targets LptA, a previously untapped bacterial protein involved in constructing the outer cell membrane. Phare Bio, a nonprofit in the Antibiotics-AI Project, is refining NG1 and DN1 to improve their drug properties, while researchers expand the AI platform to target other tough pathogens like Mycobacterium tuberculosis (the causative agent of tuberculosis) and Pseudomonas aeruginosa (a group of bacteria that often causes infections in health-care settings). The study, first published in the journal Cell, signals a hopeful turn in the global struggle against superbugs. However, these findings are early-stage. Initial tests and lab results are encouraging, but human safety and efficacy must be established through rigorous lab refinement and clinical trials, a process that could span several years. This effort builds on MIT's previous breakthroughs in AI-guided antibiotic development, including halicin, discovered in 2020 via deep learning, and abaucin, discovered in 2023 via a machine-learning algorithm.
[3]
Using generative AI, researchers design compounds that can kill drug-resistant bacteria
Caption: With help from artificial intelligence, MIT researchers have discovered novel antibiotics that can combat two hard-to-treat infections: a drug-resistant form of gonorrhea and multi-drug-resistant Staphylococcus aureus (MRSA). With help from artificial intelligence, MIT researchers have designed novel antibiotics that can combat two hard-to-treat infections: drug-resistant Neisseria gonorrhoeae and multi-drug-resistant Staphylococcus aureus (MRSA). Using generative AI algorithms, the research team designed more than 36 million possible compounds and computationally screened them for antimicrobial properties. The top candidates they discovered are structurally distinct from any existing antibiotics, and they appear to work by novel mechanisms that disrupt bacterial cell membranes. This approach allowed the researchers to generate and evaluate theoretical compounds that have never been seen before -- a strategy that they now hope to apply to identify and design compounds with activity against other species of bacteria. "We're excited about the new possibilities that this project opens up for antibiotics development. Our work shows the power of AI from a drug design standpoint, and enables us to exploit much larger chemical spaces that were previously inaccessible," says James Collins, the Termeer Professor of Medical Engineering and Science in MIT's Institute for Medical Engineering and Science (IMES) and Department of Biological Engineering. Collins is the senior author of the study, which appears today in Cell. The paper's lead authors are MIT postdoc Aarti Krishnan, former postdoc Melis Anahtar '08, and Jacqueline Valeri PhD '23. Exploring chemical space Over the past 45 years, a few dozen new antibiotics have been approved by the FDA, but most of these are variants of existing antibiotics. At the same time, bacterial resistance to many of these drugs has been growing. Globally, it is estimated that drug-resistant bacterial infections cause nearly 5 million deaths per year. In hopes of finding new antibiotics to fight this growing problem, Collins and others at MIT's Antibiotics-AI Project have harnessed the power of AI to screen huge libraries of existing chemical compounds. This work has yielded several promising drug candidates, including halicin and abaucin. To build on that progress, Collins and his colleagues decided to expand their search into molecules that can't be found in any chemical libraries. By using AI to generate hypothetically possible molecules that don't exist or haven't been discovered, they realized that it should be possible to explore a much greater diversity of potential drug compounds. In their new study, the researchers employed two different approaches: First, they directed generative AI algorithms to design molecules based on a specific chemical fragment that showed antimicrobial activity, and second, they let the algorithms freely generate molecules, without having to include a specific fragment. For the fragment-based approach, the researchers sought to identify molecules that could kill N. gonorrhoeae, a Gram-negative bacterium that causes gonorrhea. They began by assembling a library of about 45 million known chemical fragments, consisting of all possible combinations of 11 atoms of carbon, nitrogen, oxygen, fluorine, chlorine, and sulfur, along with fragments from Enamine's REadily AccessibLe (REAL) space. Then, they screened the library using machine-learning models that Collins' lab has previously trained to predict antibacterial activity against N. gonorrhoeae. This resulted in nearly 4 million fragments. They narrowed down that pool by removing any fragments predicted to be cytotoxic to human cells, displayed chemical liabilities, and were known to be similar to existing antibiotics. This left them with about 1 million candidates. "We wanted to get rid of anything that would look like an existing antibiotic, to help address the antimicrobial resistance crisis in a fundamentally different way. By venturing into underexplored areas of chemical space, our goal was to uncover novel mechanisms of action," Krishnan says. Through several rounds of additional experiments and computational analysis, the researchers identified a fragment they called F1 that appeared to have promising activity against N. gonorrhoeae. They used this fragment as the basis for generating additional compounds, using two different generative AI algorithms. One of those algorithms, known as chemically reasonable mutations (CReM), works by starting with a particular molecule containing F1 and then generating new molecules by adding, replacing, or deleting atoms and chemical groups. The second algorithm, F-VAE (fragment-based variational autoencoder), takes a chemical fragment and builds it into a complete molecule. It does so by learning patterns of how fragments are commonly modified, based on its pretraining on more than 1 million molecules from the ChEMBL database. Those two algorithms generated about 7 million candidates containing F1, which the researchers then computationally screened for activity against N. gonorrhoeae. This screen yielded about 1,000 compounds, and the researchers selected 80 of those to see if they could be produced by chemical synthesis vendors. Only two of these could be synthesized, and one of them, named NG1, was very effective at killing N. gonorrhoeae in a lab dish and in a mouse model of drug-resistant gonorrhea infection. Additional experiments revealed that NG1 interacts with a protein called LptA, a novel drug target involved in the synthesis of the bacterial outer membrane. It appears that the drug works by interfering with membrane synthesis, which is fatal to cells. Unconstrained design In a second round of studies, the researchers explored the potential of using generative AI to freely design molecules, using Gram-positive bacteria, S. aureus as their target. Again, the researchers used CReM and VAE to generate molecules, but this time with no constraints other than the general rules of how atoms can join to form chemically plausible molecules. Together, the models generated more than 29 million compounds. The researchers then applied the same filters that they did to the N. gonorrhoeae candidates, but focusing on S. aureus, eventually narrowing the pool down to about 90 compounds. They were able to synthesize and test 22 of these molecules, and six of them showed strong antibacterial activity against multi-drug-resistant S. aureus grown in a lab dish. They also found that the top candidate, named DN1, was able to clear a methicillin-resistant S. aureus (MRSA) skin infection in a mouse model. These molecules also appear to interfere with bacterial cell membranes, but with broader effects not limited to interaction with one specific protein. Phare Bio, a nonprofit that is also part of the Antibiotics-AI Project, is now working on further modifying NG1 and DN1 to make them suitable for additional testing. "In a collaboration with Phare Bio, we are exploring analogs, as well as working on advancing the best candidates preclinically, through medicinal chemistry work," Collins says. "We are also excited about applying the platforms that Aarti and the team have developed toward other bacterial pathogens of interest, notably Mycobacterium tuberculosis and Pseudomonas aeruginosa." The research was funded, in part, by the U.S. Defense Threat Reduction Agency, the National Institutes of Health, the Audacious Project, Flu Lab, the Sea Grape Foundation, Rosamund Zander and Hansjorg Wyss for the Wyss Foundation, and an anonymous donor.
[4]
Antibiotics designed with the help of AI attack bacteria in ...
New antibiotics designed with the help of AI show promise against two deadly drug-resistant bacteria. Two of the new compounds attack bacteria in ways unseen in existing drugs. Development of new antibiotics has failed to keep pace with rising antibiotic resistance in bacteria, leading to an urgent public health crisis. Once-treatable diseases are turning into relentless foes, with antibiotic-resistant bacteria linked to approximately five million deaths each year. Now, biomedical engineer James Collins from the Massachusetts Institute of Technology, US, and his team have used a deep learning model to design entirely new antibiotics. Using a graph neural network that links chemical structure to predicted properties, the researchers sifted through more than 45 million chemical fragments to identify structures with potential antibacterial activity against Neisseria gonorrhoeae and Staphylococcus aureus. After manually filtering out fragments with undesirable properties like toxicity, metabolic instability and promiscuous binding, Collins' team used a subset of promising fragments to act as seeds for two generative AI models. These models specialised in fragment-based design, in which molecules are created by modifying different side-chains, and de novo design, in which molecules are generated from scratch. The AI models designed over 36 million compounds and screened them for their potential antibacterial activity. Highly potent Collins' team then synthesised 24 of the AI-generated compounds, with two molecules, called NG1 and DN1, standing out due to their potency, selectivity and entirely new mechanisms of action. 'NG1 selectively targets pathogenic N. gonorrhoeae' while sparing beneficial commensal Neisseria species such as N. cinerea and N. mucosa, which are important for maintaining a healthy vaginal microbiome,' says Aarti Krishnan, who worked on the project. 'It also killed the highly drug-resistant strain found in the US that lost susceptibility not just to ceftriaxone but to all other drugs previously recommended for first-line treatment.' DN1 showed strong bactericidal activity against S. aureus with its potency comparable to those of other frontline antibiotics like vancomycin and linezolid. Its bactericidal activity was rapid, notes Krishnan, 'achieving complete killing within four hours, whereas vancomycin requires around ten hours'. The compound was also active against strains that are resistant to current antibiotics. Jonathan Stokes, a computational chemist at McMaster University in Canada who wasn't involved in the work, calls it an 'interesting demonstration' of generative AI's use in early-phase antibiotic discovery, 'particularly in exploring bioactive chemical space against two distinct pathogens'. However, Stokes also highlights two limitations of such AI-aided approaches. The first is that 'many AI-generated structures remain impossible to synthesise', which can result in expensive and time-consuming false starts. Secondly, the mechanism of action of AI-designed antibiotics needs to be unraveled, another technically challenging and expensive process. 'But I'm certain the field will get to where it needs to go,' he adds.
[5]
AI has produced 2 new antibiotics to kill 'superbugs'. It's promising - but we shouldn't get too excited yet
Western Sydney University provides funding as a member of The Conversation AU. Researchers from the Massachusetts Institute of Technology (MIT) have used artificial intelligence (AI) to design two new antibiotics effective against antibiotic-resistant bacteria, or "superbugs". This is a potentially exciting development, but it's important to note there are several hurdles to overcome before we might see these antibiotics used in the real world. And if this eventuates, it's likely to be some years away. So how did the researchers harness AI to develop these antibiotics? Which superbugs will they target? And what happens next? Antibiotic resistance is a global health threat Frequent overuse of antibiotics in medicine and agriculture has led to the evolution of new strains of bacteria resistant to an increasing range of antibiotics. This global public health crisis makes the development of new antibiotics a significant challenge. Antibiotic-resistant superbugs contribute to around 5 million deaths worldwide annually, and directly cause more than 1.2 million deaths. It's estimated superbug infections could lead to more than A$2.5 trillion in lost economic output globally by 2050. Antibiotic resistance is also increasingly a problem of inequity, with many poorer countries unable to access newer antibiotics to overcome resistant bacteria. Targeting 2 key superbugs The researchers used AI to design antibiotics against two prominent superbugs: Neisseria gonorrhoeae and methicillin-resistant Staphylococcus aureus (MRSA). N. gonorrhoeae causes the sexually transmitted disease gonorrhoea, which has developed high levels of resistance to antibiotics in recent years. The inability to treat it effectively has contributed to a rapid spread of the disease. There were more than 82 million new cases in 2020, mostly in developing nations. MRSA is a resistant strain of the bacteria Staphylococcus aureus (often referred to as "golden staph"). S. aureus can cause skin infections or serious blood and organ infections. Patients who get sick with the resistant MRSA strain are estimated to be 64% more likely to die as a result of an infection. To address these challenges, the MIT team harnessed generative AI in two ways. What did the researchers do? The research team trained an AI algorithm, called a machine learning neural network, using chemical structures. We can think of this as similar to the way an AI language model would be trained using words. The first approach, used for gonorrhoea, involved the algorithm screening a large database of existing compounds that had demonstrated antibiotic activity against N. gonorrhoeae. The AI algorithm then used the chemical structures of these compounds as "seeds" and built on them, generating new compounds by adding chemical structures one by one. This approach led to 80 new candidate compounds, two of which could be chemically synthesised (meaning the scientists could make them in the lab). In the end, one of these demonstrated strong effectiveness against N. gonorrhoeae. It was able to kill the bacteria on a petri dish and in a mouse model. The second approach, used for MRSA, started from scratch, prompting the AI algorithm with only simple chemical structures such as water and ammonia. The algorithm then predicted chemical structures that would interact effectively with vulnerabilities in the bacteria's cell defences, and came up with entirely new antibiotic compounds. Out of around 90 candidates, 22 were synthesised and tested in the lab. Six showed strong antibacterial activity against MRSA in a petri dish. The most promising compound successfully cleared an MRSA skin infection in a mouse model. Advantages and challenges An important element of this research is that the two new antibiotics are not just novel in their structure, but also in their mechanisms of action (in other words, how they work against the bacteria). Traditionally, antibiotic development has relied on tweaking existing antibiotics. It's hoped the fact these AI-generated molecules have entirely new mechanisms of action will make them more difficult for gonorrhoea and MRSA to evade. Prior to this research, when it comes to antibiotic development, AI has mainly been used to narrow down libraries of already existing compounds or to modify chemical structures of currently used drugs. While this work is very promising, several hurdles remain. Both antibiotics must undergo vigorous testing for safety and efficacy in humans through clinical trials, which will take several years and require significant funding. Another challenge could be financial. As these antibiotics would be intended as "last resort" drugs to preserve their effectiveness, their market use will be limited. This limits the financial incentive for pharmaceutical companies to invest in their development and production. Nevertheless, this work marks a significant milestone in drug discovery and is an example of how AI might reshape the fight against infectious diseases in the future.
[6]
Artificial intelligence enables discovery of novel compounds to fight drug-resistant bacteria
Massachusetts Institute of TechnologyAug 14 2025 With help from artificial intelligence, MIT researchers have designed novel antibiotics that can combat two hard-to-treat infections: drug-resistant Neisseria gonorrhoeae and multi-drug-resistant Staphylococcus aureus (MRSA). Using generative AI algorithms, the research team designed more than 36 million possible compounds and computationally screened them for antimicrobial properties. The top candidates they discovered are structurally distinct from any existing antibiotics, and they appear to work by novel mechanisms that disrupt bacterial cell membranes. This approach allowed the researchers to generate and evaluate theoretical compounds that have never been seen before - a strategy that they now hope to apply to identify and design compounds with activity against other species of bacteria. We're excited about the new possibilities that this project opens up for antibiotics development. Our work shows the power of AI from a drug design standpoint, and enables us to exploit much larger chemical spaces that were previously inaccessible." James Collins, the Termeer Professor of Medical Engineering and Science in MIT's Institute for Medical Engineering and Science (IMES) and Department of Biological Engineering Collins is the senior author of the study, which appears today in Cell. The paper's lead authors are MIT postdoc Aarti Krishnan, former postdoc Melis Anahtar '08, and Jacqueline Valeri PhD '23. Exploring chemical space Over the past 45 years, a few dozen new antibiotics have been approved by the FDA, but most of these are variants of existing antibiotics. At the same time, bacterial resistance to many of these drugs has been growing. Globally, it is estimated that drug-resistant bacterial infections cause nearly 5 million deaths per year. In hopes of finding new antibiotics to fight this growing problem, Collins and others at MIT's Antibiotics-AI Project have harnessed the power of AI to screen huge libraries of existing chemical compounds. This work has yielded several promising drug candidates, including halicin and abaucin. To build on that progress, Collins and his colleagues decided to expand their search into molecules that can't be found in any chemical libraries. By using AI to generate hypothetically possible molecules that don't exist or haven't been discovered, they realized that it should be possible to explore a much greater diversity of potential drug compounds. In their new study, the researchers employed two different approaches: First, they directed generative AI algorithms to design molecules based on a specific chemical fragment that showed antimicrobial activity, and second, they let the algorithms freely generate molecules, without having to include a specific fragment. For the fragment-based approach, the researchers sought to identify molecules that could kill N. gonorrhoeae, a Gram-negative bacterium that causes gonorrhea. They began by assembling a library of about 45 million known chemical fragments, consisting of all possible combinations of 11 atoms of carbon, nitrogen, oxygen, fluorine, chlorine, and sulfur, along with fragments from Enamine's REadily AccessibLe (REAL) space. Then, they screened the library using machine-learning models that Collins' lab has previously trained to predict antibacterial activity against N. gonorrhoeae. This resulted in nearly 4 million fragments. They narrowed down that pool by removing any fragments predicted to be cytotoxic to human cells, displayed chemical liabilities, and were known to be similar to existing antibiotics. This left them with about 1 million candidates. "We wanted to get rid of anything that would look like an existing antibiotic, to help address the antimicrobial resistance crisis in a fundamentally different way. By venturing into underexplored areas of chemical space, our goal was to uncover novel mechanisms of action," Krishnan says. Through several rounds of additional experiments and computational analysis, the researchers identified a fragment they called F1 that appeared to have promising activity against N. gonorrhoeae. They used this fragment as the basis for generating additional compounds, using two different generative AI algorithms. One of those algorithms, known as chemically reasonable mutations (CReM), works by starting with a particular molecule containing F1 and then generating new molecules by adding, replacing, or deleting atoms and chemical groups. The second algorithm, F-VAE (fragment-based variational autoencoder), takes a chemical fragment and builds it into a complete molecule. It does so by learning patterns of how fragments are commonly modified, based on its pretraining on more than 1 million molecules from the ChEMBL database. Those two algorithms generated about 7 million candidates containing F1, which the researchers then computationally screened for activity against N. gonorrhoeae. This screen yielded about 1,000 compounds, and the researchers selected 80 of those to see if they could be produced by chemical synthesis vendors. Only two of these could be synthesized, and one of them, named NG1, was very effective at killing N. gonorrhoeae in a lab dish and in a mouse model of drug-resistant gonorrhea infection. Additional experiments revealed that NG1 interacts with a protein called LptA, a novel drug target involved in the synthesis of the bacterial outer membrane. It appears that the drug works by interfering with membrane synthesis, which is fatal to cells. Unconstrained design In a second round of studies, the researchers explored the potential of using generative AI to freely design molecules, using Gram-positive bacteria, S. aureus as their target. Again, the researchers used CReM and VAE to generate molecules, but this time with no constraints other than the general rules of how atoms can join to form chemically plausible molecules. Together, the models generated more than 29 million compounds. The researchers then applied the same filters that they did to the N. gonorrhoeae candidates, but focusing on S. aureus, eventually narrowing the pool down to about 90 compounds. They were able to synthesize and test 22 of these molecules, and six of them showed strong antibacterial activity against multi-drug-resistant S. aureus grown in a lab dish. They also found that the top candidate, named DN1, was able to clear a methicillin-resistant S. aureus (MRSA) skin infection in a mouse model. These molecules also appear to interfere with bacterial cell membranes, but with broader effects not limited to interaction with one specific protein. Phare Bio, a nonprofit that is also part of the Antibiotics-AI Project, is now working on further modifying NG1 and DN1 to make them suitable for additional testing. "In a collaboration with Phare Bio, we are exploring analogs, as well as working on advancing the best candidates preclinically, through medicinal chemistry work," Collins says. "We are also excited about applying the platforms that Aarti and the team have developed toward other bacterial pathogens of interest, notably Mycobacterium tuberculosis and Pseudomonas aeruginosa." The research was funded, in part, by the U.S. Defense Threat Reduction Agency, the National Institutes of Health, the Audacious Project, Flu Lab, the Sea Grape Foundation, Rosamund Zander and Hansjorg Wyss for the Wyss Foundation, and an anonymous donor. Massachusetts Institute of Technology Journal reference: Krishnan, A., et al. (2025). A generative deep learning approach to de novo antibiotic design. Cell. doi.org/10.1016/j.cell.2025.07.033.
[7]
AI designs new superbug-killing antibiotics for gonorrhoea and MRSA
Researchers have previously used AI to trawl through thousands of known chemicals in an attempt to identify ones with potential to become new antibiotics. Now, the MIT team have gone one step further by using generative AI to design antibiotics in the first place for the sexually transmitted infection gonorrhoea and for potentially-deadly MRSA (methicillin-resistant Staphylococcus aureus). Their study, published in the journal Cell, interrogated 36 million compounds including those that either do not exist or have not yet been discovered. Scientists trained the AI by giving it the chemical structure of known compounds alongside data on whether they slow the growth of different species of bacteria. The AI then learns how bacteria are affected by different molecular structures, built of atoms such as carbon, oxygen, hydrogen and nitrogen. Two approaches were then tried to design new antibiotics with AI. The first identified a promising starting point by searching through a library of millions of chemical fragments, eight to 19 atoms in size, and built from there. The second gave the AI free reign from the start. The design process also weeded out anything that looked too similar to current antibiotics. It also tried to ensure they were inventing medicines rather than soap and to filter out anything predicted to be toxic to humans. Scientists used AI to create antibiotics for gonorrhoea and MRSA, a type of bacteria that lives harmlessly on the skin but can cause a serious infection if it enters the body. Once manufactured, the leading designs were tested on bacteria in the lab and on infected mice, resulting in two new potential drugs.
[8]
AI antibiotics beat superbug MRSA and gonorrhoea in lab tests
Antibiotics kill bacteria, but over time some types of bacteria have evolved to dodge the drugs' effects. Experts say overuse of antibiotics has also helped bacteria to survive certain antibiotics. This means that some bacteria are harder to treat, and there has been a shortage of new antibiotics for decades. The new discovery was made by scientists at the Massachusetts Institute of Technology (MIT) in America. The team trained the AI by giving it the chemical structure of known compounds alongside data on whether they slow the growth of different species of bacteria. The new antibiotic drugs were then put together atom by atom using the data and AI algorithms. The drugs which were invented by AI were then tested on two bacterial infections which are known to be hard to treat - and scientists found they successfully destroyed both of them. Experts say the two new antibiotics need years of testing before they can be used on humans, but that they're really excited by the results. The team say it's a huge breakthrough, and that AI could start a "second golden age" in antibiotic discovery.
[9]
AI antibiotics for superbugs could lead to 'golden age of discovery'
Artificial intelligence has created two antibiotics that could herald a "golden age" in the fight against superbugs. AI virtually invented more than 50 million compounds and investigated whether they could kill MRSA and gonorrhoea, two of the most common superbugs. More than 52,000 people a year in the UK catch antibiotic-resistant infections, which cause around 2,000 deaths annually. Antimicrobial resistance, which creates superbugs, has been called the "silent pandemic" and the problem of antibiotic resistance is set to get worse. These deadly infections occur when a bacterium is treated with a drug but works out ways of neutralising the medicine. The genetic protections make drugs less effective and can make common infections lethal. Researchers at the Massachusetts Institute of Technology (MIT) used AI to come up with completely new ways of targeting these pathogens in the hope of making a breakthrough against superbugs. "We're excited because we show that generative AI can be used to design completely new antibiotics," Prof James Collins, the leader of the project at MIT, told the BBC. "AI can enable us to come up with molecules, cheaply and quickly and in this way, expand our arsenal, and really give us a leg up in the battle of our wits against the genes of superbugs." AI was used to make as many hypothetical chemical compounds as possible that could target and kill the two bacterial infections.
[10]
MIT scientists use AI to develop new drugs for gonorrhoea and MRSA
The researchers aimed to find completely new ways of tackling antimicrobial resistance. Scientists have used artificial intelligence (AI) to create potential new drugs for so-called superbugs - stubborn bacterial infections that can evade existing treatments. AI has reshaped drug discovery in recent years, helping researchers and drugmakers pinpoint promising treatments by speeding up the painstaking process of finding effective compounds that could be turned into medicines. But the scientists from the Massachusetts Institute of Technology (MIT) went a step further, using AI to generate hypothetical chemical molecules that either haven't been discovered or don't exist yet. They aimed to find completely new ways of tackling antimicrobial resistance, which is when bacteria, viruses, fungi, or parasites evolve to the point where the drugs designed to kill them are no longer effective, making infections harder to treat. The MIT team targeted drug-resistant gonorrhoea, which US health officials call an "urgent public health threat," and multi-drug resistant Staphylococcus aureus (MDRSA). That includes methicillin-resistant Staphylococcus aureus (MRSA), which people can acquire through contact with infected people or contaminated medical equipment. "We wanted to get rid of anything that would look like an existing antibiotic, to help address the antimicrobial resistance (AMR) crisis in a fundamentally different way," Aarti Krishnan, an MIT researcher and one of the study's authors, said in a statement. "By venturing into underexplored areas of chemical space, our goal was to uncover novel mechanisms of action," Krishnan added. The team used generative AI algorithms to create more than 36 million potential compounds and then find the best candidates to kill the bacteria. They identified a fragment that appeared to work well against gonorrhoea bacteria - and after some additional fine-tuning, they developed two of these digital candidates into actual compounds. One of them, which they named NG1, was highly effective at killing gonorrhoea bacteria in a lab dish and a mouse model. After a similar process to find potential treatments for MDRSA, six molecules appeared effective against bacteria that was grown in a lab dish. The researchers said the findings, which were published in the journal Cell, could help them create and evaluate potential new compounds to target other species of bacteria. Globally, drug-resistant bacterial infections contributed to an estimated 4.71 million deaths in 2021, and that figure is expected to rise in the coming decades. "Our work shows the power of AI from a drug design standpoint, and enables us to exploit much larger chemical spaces that were previously inaccessible," James Collins, an MIT professor and one of the study's authors, said in a statement. The scientists are now working with Phare Bio, a nonprofit biotech company, to continue testing compounds in the lab. If they continue to show promise, these drug candidates could eventually be tested in clinical trials. "We're excited about the new possibilities that this project opens up for antibiotics development," Collins said.
[11]
AI used to design antibiotics that can combat drug-resistant superbugs gonorrhoea and MRSA
Antibiotics are used to kill bacteria, but some infections have become resistant to drugs. It is estimated drug-resistant bacterial infections cause nearly 5 million deaths per year worldwide. New antibiotics that could kill drug-resistant gonorrhoea and MRSA have been developed with the help of artificial intelligence (AI), researchers have said. A team at Massachusetts Institute of Technology (MIT) used generative AI algorithms to design more than 36 million possible compounds. Once computationally screened for antimicrobial properties, the top candidates were shown to be structurally different from any existing antibiotics. They also seemed to work in a new way - by disrupting bacterial cell membranes. Antibiotics kill bacteria, but some infections have become resistant to drugs. It is estimated that drug-resistant bacterial infections cause nearly 5 million deaths per year worldwide. Two compounds were found to be effective against gonorrhoea and MRSA infections - namely NG1 and DN1 respectively. A non-profit organisation is now working on modifying the compounds to make them suitable for further testing. The research appeared on Thursday in scientific journal Cell. MIT Professor James Collins, the paper's senior author, said: "We're excited about the new possibilities that this project opens up for antibiotics development. "Our work shows the power of AI from a drug design standpoint, and enables us to exploit much larger chemical spaces that were previously inaccessible." Read more from Sky News: What do Ukrainians think of Trump-Putin summit? 'Zombie rabbit' pictures explained One of the study's lead authors, MIT postdoc Aarti Krishnan, said: "We wanted to get rid of anything that would look like an existing antibiotic, to help address the antimicrobial resistance crisis in a fundamentally different way. "By venturing into underexplored areas of chemical space, our goal was to uncover novel mechanisms of action."
[12]
AI designs new antibiotics to combat drug-resistant superbugs
As the global health crisis of antibiotic resistance continues to grow, causing more than a million deaths each year, researchers are turning to artificial intelligence to create entirely new weapons against superbugs. According to a recent report by the BBC's health and science correspondent, James Gallagher, a team at the Massachusetts Institute of Technology (MIT) has used generative AI to invent two potential new antibiotics from scratch. The study, published in the journal Cell, details how these AI-designed compounds have successfully killed drug-resistant gonorrhoea and MRSA in laboratory and animal tests, offering a potential breakthrough in a field that has seen a shortage of new drugs for decades. Unlike previous approaches that used AI to screen thousands of existing chemicals for antibiotic potential, the MIT team has taken a significant step forward by using generative AI to design completely new molecules atom-by-atom. To achieve this, the researchers first trained their AI model by providing it with the chemical structures of known compounds along with data on whether they were effective at slowing the growth of different species of bacteria. This process allowed the AI to learn how different molecular arrangements of atoms like carbon, oxygen, and hydrogen affect bacteria. Once trained, the AI interrogated a massive dataset of 36 million chemical compounds, including many that either do not yet exist or have not been discovered. The researchers then used two different methods to generate novel antibiotic candidates. The first approach involved identifying a promising chemical fragment from a library and then building upon it, while the second method gave the AI free rein to design a new molecule from the start. The design process also included several important constraints to ensure the outputs were viable. The AI was programmed to weed out any designs that looked too similar to existing antibiotics, to filter out anything predicted to be toxic to humans, and to ensure it was inventing medicines rather than substances like soap. The AI-driven design process resulted in two new potential drugs specifically targeting the sexually transmitted infection gonorrhoea and methicillin-resistant Staphylococcus aureus, or MRSA, a bacteria that can cause serious infections. When these compounds were manufactured, they were successfully tested on bacteria in the lab and on infected mice. Professor James Collins, one of the MIT researchers, stated that this work shows that generative AI can be used to come up with new molecules cheaply and quickly, potentially starting a "second golden age" in antibiotic discovery. Despite these promising early results, the path to clinical use is long and filled with challenges. The two compounds are not ready for human trials and will require an estimated one to two more years of refinement before the lengthy process of testing them in people could even begin. A major hurdle is the difficulty of manufacturing the complex molecules designed by the AI. In the case of the gonorrhoea treatments, of the top 80 compounds designed in theory, researchers were only able to successfully synthesize two of them in the lab. Beyond the scientific and manufacturing challenges, there is also a significant economic problem facing antibiotic development. If a new antibiotic were invented, its use would need to be restricted to preserve its effectiveness against evolving bacteria. This necessary caution makes it difficult for pharmaceutical companies to turn a profit on their investment. Furthermore, experts in the field note that AI drug discovery models still need to be improved. Professor Collins called for "better models" that can more accurately predict how a drug will perform in the human body, not just in a laboratory setting.
[13]
Artificial Intelligence Designs Two Potential Antibiotics to Combat Drug-Resistant Gonorrhoea and MRSA
Researchers have used AI to design two promising antibiotics that are helpful in combating drug-resistant bacteria, including gonorrhoea and MRSA. AI predicted efficient molecular structures, which were validated in laboratory tests. This innovative method could revolutionize antibiotic discovery, providing faster, cost-efficient development of treatments against superbugs and addressing one of the most urgent public health threats. In a groundbreaking development, researchers at the Massachusetts Institute of Technology (MIT) have harnessed artificial intelligence (AI) to design two new innovative antibiotics capable of fighting drug-resistant superbugs, including gonorrhoea and MRSA. The method makes a significant step forward in addressing the growing global threat caused by antimicrobial resistance (AMR), which has been explained by the World Health Organization as one of the most urgent public health challenges of the 21st century. One of the newly developed antibiotics has shown remarkable progress against MRSA (methicillin-resistant Staphylococcus aureus), a dangerous pathogen responsible for severe hospital-acquired infections. MRSA is notorious for evading standard antibiotics, leading to prolonged illnesses and higher mortality rates. The second compound concentrates on drug-resistant strains of Neisseria gonorrhoeae, the bacterium responsible for gonorrhoea. Rising cases of antibiotic-resistant gonorrhoea have raised serious concerns about treatment failures and broader public health risks. Laboratory tests confirmed that both compounds successfully killed the targeted bacteria, even those resistant to multiple existing medications. While these results are preliminary, they provide a strong base for further preclinical and clinical testing. The successful use of AI in developing these antibiotics signals a new era in drug discovery. By reducing the time and cost involved with developing new drugs, AI could enhance the response to emerging superbugs and other infectious diseases. This is particularly important as the antibiotic pipeline has slowed in recent years, making healthcare systems vulnerable to resistant infections. Experts say that AI-designed antibiotics are not a complete solution but rather an important tool in the ongoing battle against AMR. Complementary strategies, including proper antibiotic stewardship, infection prevention, and global surveillance, remain crucial to handling the spread of resistant bacteria. Researchers aim to advance the new compounds into further preclinical trials to evaluate their safety, efficacy, and side effects in humans. If successful, these antibiotics could provide new treatment for patients affected by resistant infections and save countless lives globally. Q1. What are superbugs? A1. Superbugs are bacteria resistant to multiple antibiotics, making infections harder to treat. They pose a serious global health risk. Q2. How did AI help in developing these antibiotics? A2. AI analyzed vast chemical data to predict molecular structures likely to combat resistant bacteria effectively. (You can now subscribe to our Economic Times WhatsApp channel)
[14]
MIT Researchers Craft New Molecules With Gen AI to Create Superbug Antibiotics | PYMNTS.com
By completing this form, you agree to receive marketing communications from PYMNTS and to the sharing of your information with our sponsor, if applicable, in accordance with our Privacy Policy and Terms and Conditions. MRSA, or Methicillin-resistant Staphylococcus aureus, is caused by a staph bacteria that has become resistant to most antibiotics, according to the Centers for Disease Control. Harvard Medical School found that "MRSA proves to be especially adept at evading the grasp of antibiotics, becoming a truly dangerous superbug." Common places to contract MRSA are hospitals, long-term care facilities and communities, according to the National Institutes of Health (NIH). Traditional methods to discover new antibiotics means scientists look through existing chemical databases of molecules that have been found in nature or synthetically built compounds, then test them against bacteria. But those resources are mostly variations of known molecules, which makes it harder to discover new classes of drugs, according to the MIT researchers' paper, which was published in the journal Cell. The MIT team used gen AI to imagine completely new chemical structures that have never been created before. The AI models they used learned patterns from millions of known molecules, then remixed them. "We used generative AI to create antibiotics that didn't yet exist to come up with molecules can act in novel ways and therefore can overcome existing resistance mechanisms," MIT professor James Collins, senior author of the study, told PYMNTS. According to PYMNTS Intelligence data, the gen AI market in healthcare is expected to reach $22 billion by 2032 as the healthcare industry taps into AI's potential. AI is reinventing drug discovery. According to the NIH, one of the main challenges in drug discovery is the "vast chemical space" that must be explored to identify potential drug candidates. "Traditional methods for screening large compound libraries are labor-intensive, time-consuming, and often result in a limited number of hits," the NIH said in a report. "AI-driven virtual screening approaches leverage machine learning algorithms that can rapidly sift through vast datasets of chemical compounds as well as predict their biological activity against specific drug targets." Using AI can shorten the time it takes from identifying promising drugs to market release, which typically takes 15 years, according to the NIH. At MIT, researchers used AI to sift through more than 29 million molecules to narrow it down to 22 that were most likely to work and also can be realistically synthesized. Six of them showed promise, and one candidate, DN1, was especially effective in clearing MRSA from mice. MIT researchers used the same procedure to find promising antibiotics to fight multidrug-resistant Neisseria gonorrhoeae, the bacteria that leads to gonorrhea, a common sexually transmitted infection. Out of 45 million in the database, the team used AI to narrow it down to two that could be synthesized. Only one was effective: NG1. The authors noted that gen AI lets scientists tap vast molecular possibilities that may yield new drug classes. With antibiotic resistance causing nearly five million deaths annually worldwide, the team wrote, the approach may help revive a field long neglected by large pharmaceutical companies. They said that between 1980 and 2003, only five antibacterial agents were developed by the top 15 drugmakers. The team is part of MIT's Antibiotics-AI Project that was created to fill in the gap left by big pharmaceutical companies. Their partner in this study, the nonprofit Phare Bio, is further modifying DN1 and NG1 to ready them for further testing. Phare Bio is also part of MIT's antibiotics program. Asked when these antibiotics will be ready for the public, Collins said: "We expect it will be several years before AI-designed molecules are approved for public use. We are working with a non-profit, Phare Bio, to optimize and advance the most promising molecules to the clinic." Read more: Can AI Cure Cancer? Experts Say the Answer Is Complicated Stanford AI Agents' Lab Finds Promising COVID-19 Drug Leads in Days
[15]
How Artificial Intelligence is Fighting Deadly Infections with New Antibiotics?
These breakthroughs could spark a revival in antibiotic research, offering powerful new options for global health. The world is running out of answers to deadly diseases as old antibiotics continue to lose their power. For decades, new drugs have barely trickled into the pipeline. Powerful systems that can explore, design, and test medicines faster than any lab could dream of are being used to combat this. The result is a new generation of antibiotics that look nothing like the past. Let's take a look at how artificial intelligence has reignited the fight against dangerous infections.
[16]
MIT scientists used AI to design antibiotics that kill Superbugs: Here's how
Breakthrough study shows AI can explore vast chemical spaces to fight drug-resistant bacteria In a breakthrough that could reshape the fight against antibiotic-resistant bacteria, MIT researchers have harnessed generative artificial intelligence (AI) to design novel compounds capable of killing drug-resistant superbugs like methicillin-resistant Staphylococcus aureus (MRSA) and Neisseria gonorrhoeae, the bacteria behind gonorrhea. Published in Cell on August 14, 2025, their work signals a new era in antibiotic discovery, offering hope against a global crisis that claims nearly 5 million lives annually. Here's how they did it. Also read: Microsoft's medical AI system is four times more accurate than human doctors: Here's how Antibiotic resistance is one of humanity's most pressing challenges. Bacteria like MRSA, which causes severe skin and bloodstream infections, and N. gonorrhoeae, increasingly untreatable due to resistance, are outpacing our ability to develop new drugs. Traditional antibiotic discovery is slow, expensive, and often produces compounds too similar to existing ones, which bacteria quickly overcome. With the World Health Organization warning that superbugs could cause 10 million deaths annually by 2050, the need for innovation is urgent. Enter the MIT team, led by James Collins, the Termeer Professor of Medical Engineering and Science in MIT's Institute for Medical Engineering and Science (IMES) and Department of Biological Engineering, with lead authors Aarti Krishnan, Melis Anahtar, and Jacqueline Valeri. Their solution: use generative AI to explore vast "chemical spaces" and create entirely new molecules with unique mechanisms to outsmart resistant bacteria. The researchers employed two distinct AI-driven approaches to design antibiotics, showcasing the technology's versatility. For Neisseria gonorrhoeae, the team started with a library of 45 million chemical fragments. Machine-learning models, trained to predict antibacterial activity, screened these fragments to identify a promising candidate dubbed F1. This fragment became the foundation for two generative AI algorithms: Chemically Reasonable Mutations (CReM) and Fragment-based Variational Autoencoder (F-VAE). These tools generated 7 million F1-containing compounds, which were then filtered for efficacy, safety, and novelty, ensuring they wouldn't be easily defeated by existing resistance mechanisms. After rigorous screening, only two compounds could be synthesized in the lab. One, named NG1, proved exceptional. In laboratory tests, NG1 killed drug-resistant N. gonorrhoeae by targeting LptA, a protein critical for bacterial membrane synthesis. This novel mechanism makes it harder for the bacteria to evolve resistance, offering a significant advantage over traditional antibiotics. For MRSA, the team took a freer approach. Instead of anchoring their design to a specific fragment, they let the AI generate 29 million compounds from scratch, guided only by basic chemical rules. Machine-learning models then filtered these for antibacterial activity and safety, narrowing the pool to 22 compounds that could be synthesized. Six showed strong activity, with the top performer, DN1, clearing MRSA infections in mouse models. DN1 works by disrupting bacterial cell membranes in a unique way, distinct from existing antibiotics, making it a powerful new weapon. Also read: What is cloud seeding? How Delhi is getting its first artificial rain The MIT team's AI-driven approach is a departure from traditional drug discovery. By generating and screening millions of compounds computationally, AI drastically reduces the time and cost of finding viable candidates. More importantly, it produces molecules with novel structures and mechanisms, like NG1's targeting of LptA or DN1's membrane-disrupting action. These fresh approaches make it tougher for bacteria to adapt, addressing a key challenge in combating resistance. "This approach allows us to explore chemical spaces that are impractical to search with conventional methods," Collins explains. "We're discovering new classes of antibiotics that bacteria haven't seen before." While NG1 and DN1 are promising, they're not ready for the pharmacy yet. The compounds require further optimization to enhance their potency and ensure safety for human use. Clinical trials, which could take years, are the next step. But the MIT team is already looking forward. In collaboration with Phare Bio, a nonprofit focused on antibiotic development, they're refining NG1 and DN1 and plan to apply their AI platform to other deadly pathogens, such as Mycobacterium tuberculosis and Pseudomonas aeruginosa. "We're just beginning to tap into AI's potential," says Krishnan, one of the lead researchers. "This platform could accelerate the discovery of antibiotics for a wide range of resistant bacteria." The MIT study, supported by the National Institutes of Health, the Broad Institute, and others, is a powerful example of how AI can tackle humanity's biggest challenges. As superbugs continue to threaten global health, innovations like these offer a lifeline. By combining cutting-edge technology with scientific ingenuity, the MIT team led by Collins and driven by researchers like Krishnan, Anahtar, and Valeri is paving the way for a future where antibiotic resistance may no longer hold medicine hostage. For now, NG1 and DN1 are early victories in a long battle. But in the labs of MIT, AI is proving to be a formidable ally in the fight to save lives from the growing threat of superbugs.
Share
Share
Copy Link
MIT researchers use generative AI to create novel antibiotics effective against drug-resistant gonorrhea and MRSA, opening new possibilities in the fight against antibiotic resistance.
AI-Powered Antibiotic Discovery
Researchers at the Massachusetts Institute of Technology (MIT) have made a significant breakthrough in the fight against antibiotic-resistant bacteria by harnessing the power of artificial intelligence (AI) to design novel antibiotics. The team, led by James Collins, used generative AI algorithms to create and screen over 36 million potential compounds, resulting in two promising antibiotics effective against drug-resistant Neisseria gonorrhoeae and methicillin-resistant Staphylococcus aureus (MRSA)
1
2
3
.
Source: MIT
The AI-Driven Approach
The researchers employed two AI-driven strategies to generate potential antibiotic candidates:
-
Fragment-based design: Starting with a chemical fragment showing promise against gonorrhea, the AI produced millions of derivatives, ultimately leading to the compound NG1
3
. -
Unconstrained generation: The AI was given free rein to design molecules targeting MRSA, resulting in the compound DN1
3
.
These approaches allowed the team to explore chemical spaces previously inaccessible through traditional drug discovery methods, potentially opening new avenues for antibiotic development
1
.Promising Results
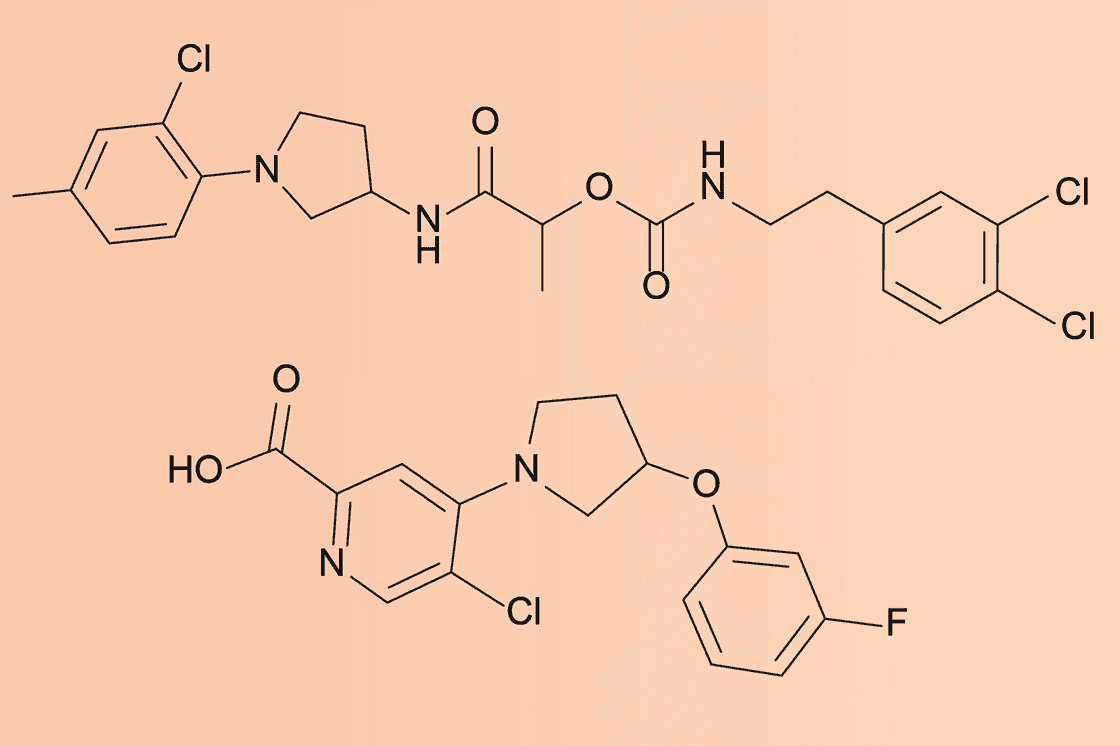
Both NG1 and DN1 showed significant efficacy in laboratory tests and animal models:
- NG1 successfully treated drug-resistant gonorrhea in cell cultures and mice, targeting a novel bacterial protein called LptA involved in outer membrane synthesis
3
4
. - DN1 demonstrated strong bactericidal activity against MRSA, achieving complete killing within four hours, compared to ten hours for vancomycin
4
.
Source: Chemistry World
Importantly, these new compounds are structurally distinct from existing antibiotics and appear to work through novel mechanisms, potentially making it more difficult for bacteria to develop resistance
2
4
.Related Stories
Challenges and Future Prospects
While the results are promising, several hurdles remain before these AI-designed antibiotics can be used in clinical settings:
-
Synthetic feasibility: Only a small fraction of the AI-generated compounds could be synthesized in the lab, highlighting a significant challenge in translating computational designs into real-world drugs
1
4
. -
Clinical trials: Rigorous testing for safety and efficacy in humans will be required, a process that could take several years and require substantial funding
5
. -
Economic considerations: As these antibiotics would likely be reserved as last-resort drugs, their limited market use could discourage pharmaceutical companies from investing in their development
5
.
Broader Implications

Source: The Conversation
This research demonstrates the potential of AI in drug discovery, particularly in exploring vast chemical spaces and identifying novel mechanisms of action. It represents a significant step forward in combating antibiotic resistance, a global health crisis responsible for millions of deaths annually
5
.The success of this approach has inspired researchers to expand their efforts to target other challenging pathogens, such as Mycobacterium tuberculosis and Pseudomonas aeruginosa
2
. As the field progresses, it is hoped that AI-driven antibiotic discovery will play a crucial role in addressing the growing threat of antimicrobial resistance and developing new weapons against superbugs1
5
.References
Summarized by
Navi
[4]
Related Stories
AI Model Predicts Antibiotic Resistance in Bacteria with High Accuracy
03 Apr 2025•Science and Research
Australian Scientists Use AI to Create Protein That Kills Antibiotic-Resistant E. coli
10 Jul 2025•Science and Research

AI Shows Promise in Detecting Antibiotic Resistance, but Human Expertise Still Crucial
18 Oct 2024•Health

Recent Highlights
1
OpenAI Releases GPT-5.4, New AI Model Built for Agents and Professional Work
Technology

2
Anthropic takes Pentagon to court over unprecedented supply chain risk designation
Policy and Regulation

3
Meta smart glasses face lawsuit and UK probe after workers watched intimate user footage
Policy and Regulation