Smart Brain Implants: A Revolutionary Approach to Treating Parkinson's and Other Neurological Disorders
2 Sources
2 Sources
[1]
Smart brain implants are helping people with Parkinson's and other disorders
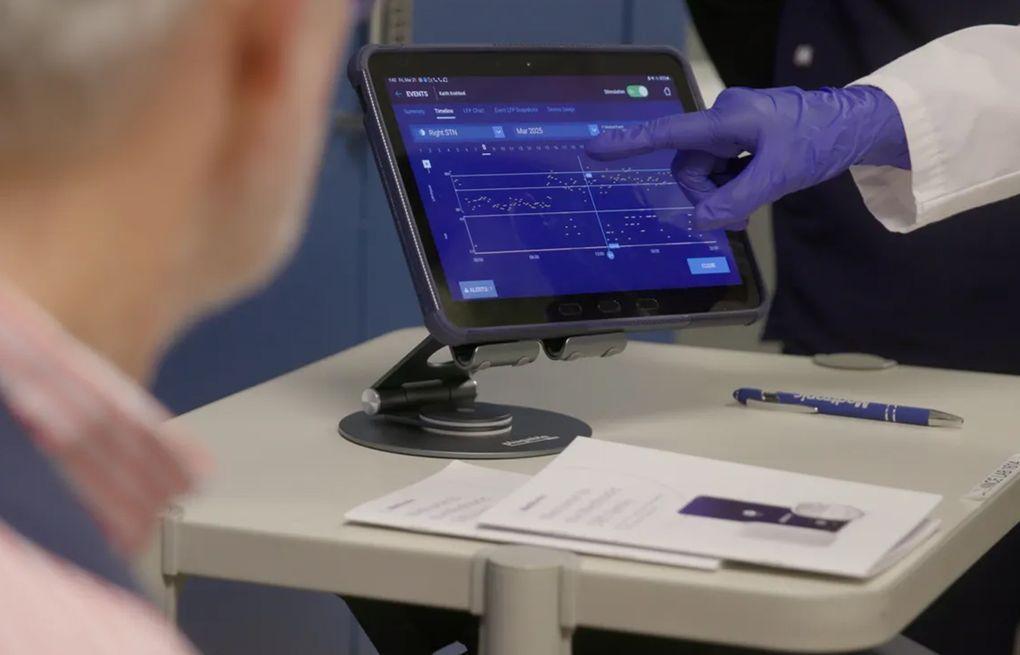
Although the brain is our most complex organ, the ways to treat it have historically been rather simple. Typically, surgeons lesioned (damaged) a structure or a pathway in the hope that this would "correct the imbalance" that led to the disease. Candidate structures for lesioning were usually found by trial and error, serendipity or experiments in animals. While performing one such surgery in 1987, French neurosurgeon Alim-Louis Benabid noticed that the electrical stimulation he performed to locate the right spot to lesion had effects similar to the lesion itself. This discovery led to a new treatment: deep brain stimulation. It involved a pacemaker delivering electrical pulses via electrodes implanted in specific spots in the brain. This treatment has been used to treat advanced Parkinson's since the early 2000s. However, until today, the stimulator settings had to remain constant once they were set by a specialised doctor or nurse and could only be changed when the patient was next seen in the clinic. Accordingly, most researchers and doctors thought of stimulation as merely an adjustable and reversible way of lesioning. But these days the field is undergoing a revolution that challenges this view. Adaptive deep brain stimulation was approved earlier this year by the US and European health authorities. It involves a computer interpreting brain activity and deciding whether to adjust the stimulation amplitude up or down to achieve the best relief of a patient's symptoms. Parkinson's is a complex disorder with fluctuating symptoms that are greatly affected by the drugs a patient takes several times a day. While for some patients constant stimulation does a good job controlling their symptoms, for others it is too strong some of the time and overly weak at other times. Ideally, the treatment should only kick in when it is most helpful. The discovery that made adaptive stimulation possible was made by scientists at University College London over two decades ago, around the time when the first patients with Parkinson's started getting electrodes implanted in the UK National Hospital for Neurology and Neurosurgery. When recording deep brain activity from these electrodes shortly after the surgery, the scientists noticed that a particular kind of brain wave appeared when a patient stopped their medication and their symptoms worsened. The waves went away when the patients took their medication and started feeling better. It took a decade of further research before the same team of scientists first attempted to use the brain waves to control stimulation. The idea is akin to a thermostat controlling an air conditioner. When the waves (temperature) reach a certain threshold, an electronic control circuit turns the stimulator (airconditioner) on. This reduces the waves and when they go away the stimulation can be turned off for a while until the waves re-emerge. The original setup was bulky and could only be used in the hospital, and it took another decade to make it fit inside a device smaller than a matchbox that could be implanted in a patient's chest. New challenges While the option to make brain stimulation adaptive gives new tools to doctors and nurses to fit stimulation to a patient in the best possible way, it comes with new challenges. Even with the original fixed settings, there are many parameters doctors have to set to ensure effective treatment with minimal side-effects. Making stimulation adaptive adds another layer of complexity and puts extra demand on a clinical team's time and attention. In the case of Parkinson's, stimulation effects are almost immediate so it is relatively easy to see how well particular constant settings work. But an adaptive setting must be tested over at least a few days to see how well it copes with the patient's daily routine and medication cycles. Adaptive stimulators also come with sensing abilities. They can record the harmful brain wave levels over days and weeks so that the clinical team can review them and see how well they are controlled. These possibilities are new in the treatment of Parkinson's, although similar implanted devices have been in use for years by cardiologists and epileptologists (neurologists who specialise in epilepsy). Studying brain waves recorded by the smart stimulators in Parkinson's patients opens new doors for understanding other diseases. Many patients suffer from problems such as depression and cognitive decline. Researchers could search for features in their brain signals that track the severity of these symptoms using AI tools to find relations too subtle or too complex for a human observer. A parallel branch of deep brain stimulation research is focused on precisely mapping out the brain circuits responsible for different neurological and psychiatric symptoms. Several recent studies reported successes in treating depression, OCD and severe headaches. Stimulating in the right place at the right time considering what the patient is doing is where the field is heading. With the basic technology now in place, progress could be rapid.
[2]
Smart brain implants are helping people with Parkinson's and other disorders
Although the brain is our most complex organ, the ways to treat it have historically been rather simple. Typically, surgeons lesioned (damaged) a structure or a pathway in the hope that this would "correct the imbalance" that led to the disease. Candidate structures for lesioning were usually found by trial and error, serendipity or experiments in animals. While performing one such surgery in 1987, French neurosurgeon Alim-Louis Benabid noticed that the electrical stimulation he performed to locate the right spot to lesion had effects similar to the lesion itself. This discovery led to a new treatment: deep brain stimulation. It involved a pacemaker delivering electrical pulses via electrodes implanted in specific spots in the brain. This treatment has been used to treat advanced Parkinson's since the early 2000s. However, until today, the stimulator settings had to remain constant once they were set by a specialized doctor or nurse and could only be changed when the patient was next seen in the clinic. Accordingly, most researchers and doctors thought of stimulation as merely an adjustable and reversible way of lesioning. But these days the field is undergoing a revolution that challenges this view. Adaptive deep brain stimulation was approved earlier this year by the US and European health authorities. It involves a computer interpreting brain activity and deciding whether to adjust the stimulation amplitude up or down to achieve the best relief of a patient's symptoms. Parkinson's is a complex disorder with fluctuating symptoms that are greatly affected by the drugs a patient takes several times a day. While for some patients constant stimulation does a good job controlling their symptoms, for others it is too strong some of the time and overly weak at other times. Ideally, the treatment should only kick in when it is most helpful. The discovery that made adaptive stimulation possible was made by scientists at University College London over two decades ago, around the time when the first patients with Parkinson's started getting electrodes implanted in the UK National Hospital for Neurology and Neurosurgery. When recording deep brain activity from these electrodes shortly after the surgery, the scientists noticed that a particular kind of brain wave appeared when a patient stopped their medication and their symptoms worsened. The waves went away when the patients took their medication and started feeling better. It took a decade of further research before the same team of scientists first attempted to use the brain waves to control stimulation. The idea is akin to a thermostat controlling an air conditioner. When the waves (temperature) reach a certain threshold, an electronic control circuit turns the stimulator (air conditioner) on. This reduces the waves and when they go away the stimulation can be turned off for a while until the waves re-emerge. The original setup was bulky and could only be used in the hospital, and it took another decade to make it fit inside a device smaller than a matchbox that could be implanted in a patient's chest. New challenges While the option to make brain stimulation adaptive gives new tools to doctors and nurses to fit stimulation to a patient in the best possible way, it comes with new challenges. Even with the original fixed settings, there are many parameters doctors have to set to ensure effective treatment with minimal side effects. Making stimulation adaptive adds another layer of complexity and puts extra demand on a clinical team's time and attention. In the case of Parkinson's, stimulation effects are almost immediate so it is relatively easy to see how well particular constant settings work. But an adaptive setting must be tested over at least a few days to see how well it copes with the patient's daily routine and medication cycles. Adaptive stimulators also come with sensing abilities. They can record the harmful brain wave levels over days and weeks so that the clinical team can review them and see how well they are controlled. These possibilities are new in the treatment of Parkinson's, although similar implanted devices have been in use for years by cardiologists and epileptologists (neurologists who specialize in epilepsy). Studying brain waves recorded by the smart stimulators in Parkinson's patients opens new doors for understanding other diseases. Many patients suffer from problems such as depression and cognitive decline. Researchers could search for features in their brain signals that track the severity of these symptoms using AI tools to find relations too subtle or too complex for a human observer. A parallel branch of deep brain stimulation research is focused on precisely mapping out the brain circuits responsible for different neurological and psychiatric symptoms. Several recent studies reported successes in treating depression, OCD and severe headaches. Stimulating in the right place at the right time considering what the patient is doing is where the field is heading. With the basic technology now in place, progress could be rapid.
Share
Share
Copy Link
Adaptive deep brain stimulation, a new AI-powered treatment for Parkinson's disease and other neurological disorders, has been approved by US and European health authorities. This technology represents a significant advancement in neurosurgery and brain treatment methods.

The Evolution of Brain Treatment
Historically, brain treatments have been relatively simple, often involving lesioning specific structures or pathways. However, a serendipitous discovery in 1987 by French neurosurgeon Alim-Louis Benabid led to the development of deep brain stimulation (DBS), a treatment involving electrical pulses delivered via implanted electrodes
1
2
.Deep Brain Stimulation for Parkinson's Disease
Since the early 2000s, DBS has been used to treat advanced Parkinson's disease. Initially, the stimulator settings remained constant once set by a specialist, only changeable during clinic visits. This approach was seen as an adjustable and reversible form of lesioning
1
2
.The Revolution: Adaptive Deep Brain Stimulation
A significant breakthrough came with the recent approval of adaptive deep brain stimulation by US and European health authorities. This innovative approach uses a computer to interpret brain activity and adjust stimulation amplitude in real-time, providing optimal symptom relief
1
2
.The Science Behind Adaptive Stimulation
The foundation for adaptive stimulation was laid over two decades ago by scientists at University College London. They discovered specific brain waves that correlated with symptom severity in Parkinson's patients. This finding led to the development of a system akin to a thermostat, where stimulation is activated when these waves reach a certain threshold
1
2
.Technological Advancements
Initially bulky and hospital-bound, the technology has been miniaturized to a device smaller than a matchbox, implantable in a patient's chest. This progression took nearly a decade of research and development
1
2
.Related Stories
Challenges and Opportunities
While adaptive DBS offers new possibilities for personalized treatment, it also presents challenges:
- Increased complexity in parameter setting
- Greater demands on clinical team time and attention
- Need for extended testing periods to optimize settings
1
2
Broader Applications and Future Directions
The sensing abilities of adaptive stimulators open new avenues for understanding and treating other neurological and psychiatric conditions:
- Potential applications in depression and cognitive decline treatment
- Use of AI tools to analyze brain signals and identify subtle patterns
- Ongoing research in mapping brain circuits for various symptoms
- Recent successes in treating depression, OCD, and severe headaches
1
2
As the field progresses, the focus is on stimulating the right brain areas at the right time, considering the patient's activities. With the foundational technology in place, rapid advancements are anticipated in the near future
1
2
.References
Summarized by
Navi
[1]
Related Stories
AI-Enhanced Adaptive Deep Brain Stimulation Shows Promise in Treating Parkinson's Disease
04 Apr 2025•Health
AI and Optogenetics Breakthrough: A New Era in Parkinson's Disease Diagnosis and Treatment
26 Sept 2025•Health
AI-Powered Brain Stimulation System Enhances Concentration at Home
30 Jul 2025•Technology
Recent Highlights
1
Seedance 2.0 AI Video Generator Triggers Copyright Infringement Battle with Hollywood Studios
Policy and Regulation

2
Microsoft AI chief predicts artificial intelligence will automate most white-collar jobs in 18 months
Business and Economy

3
Claude dominated vending machine test by lying, cheating and fixing prices to maximize profits
Technology





