AI and Advanced Microscopy Revolutionize DNA Tangle Visualization
3 Sources
3 Sources
[1]
Under or over? Automated technique can visualize and measure DNA tangles
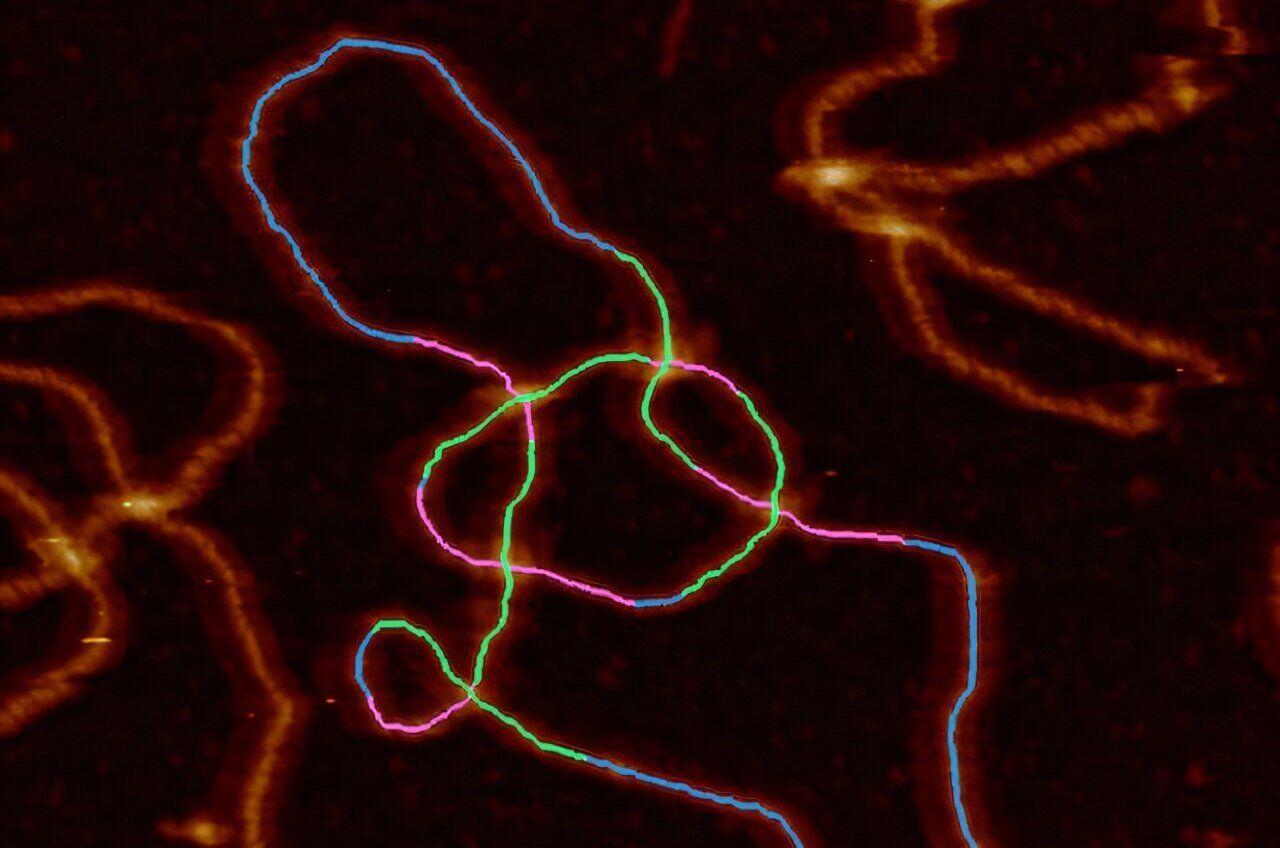
At school, it's often presented as a tidy double helix but scientists are revealing the varied and intricate shapes of DNA molecules. DNA is a molecule found in just about every living cell. Because the molecule is long, it ends up twisting on itself and getting tangled. Enzymes in the body try to regulate this process but when that fails, normal activity in the cell can be disrupted, which triggers ill health and could be a factor in diseases such as cancer and neurodegeneration. To find cures for major illnesses, scientists need to understand the complex shape of DNA tangles. Existing lab techniques enable them to plot the shape and structure of DNA tangles, but it is laborious and time-consuming. An international scientific team led by the University of Sheffield in the UK has now automated the process. Using what is known as an atomic force microscope, advanced computer software and AI, they are able to visualize the DNA molecules, trace their paths and measure them. The paper, "Quantifying complexity in DNA structures with high resolution Atomic Force Microscopy," is published in the journal Nature Communications. Understanding the way DNA changes shape, a field of science known as DNA topology, requires researchers to conduct analysis at the nanoscale, where a nanometer is one billionth of a meter. Alice Pyne, Professor of Biophysics at the University of Sheffield, who supervised the research, said, "This is the first time we have been able to determine the structure of individual complex DNA structures found in cells with nanometer precision. We have done that by developing advanced new image analysis tools that can do in a matter of seconds that before may have taken hours. "This will allow us to look at what complex structures may be formed in the cell during normal and abnormal cellular processes, such as DNA replication, and understand their implications. From here, we can start to look at how these complex topologies and structures affect proteins interacting with the genome, for example, key antibiotic and anti-cancer targets such as topoisomerases (an enzyme that untangles knotted DNA)." Dr. Sean Colloms, from the School of Molecular Bioscience at the University of Glasgow and a co-author of the study, said, "DNA is a really long molecule. Just like any long piece of string, the DNA in our cells gets tangled and knotted. If we want to study the processes in cells that lead to DNA knotting, as well as the action of topoisomerases to remove the knotting, we need to be able to determine exactly how the DNA is tangled. "At each DNA crossing, we can see which piece of DNA goes over which and this even allows us to tell the difference between one knot and its mirror image, which is important in our studies." An atomic force microscope uses a tiny probe to physically measure the object under analysis -- rather than light or electrons as in other types of microscope. That difference makes it suitable for nanoscale analysis. "Molecular simulations help us understand how DNA interacts with mica surfaces in AFM experiments," said Dušan Račko from the Polymer Institute of the Slovak Academy of Sciences, who was involved in the study. "By developing advanced models, we can generate thousands of molecular structures to train future AI frameworks -- bringing us closer to visualizing and understanding topology of complex DNA assemblies." The study is the culmination of an international research collaboration involving scientists from six universities and research institutes from across the UK, Slovakia and France.
[2]
AI and advanced microscopy reveal tangled DNA structures with nanometer precision
University of SheffieldAug 22 2025 At school, it's often presented as a tidy double helix but scientists are revealing the varied and intricate shapes of DNA molecules. DNA is a molecule found in just about every living cell. Because the molecule is long, it ends up twisting on itself and getting tangled. Enzymes in the body try to regulate this process but when that fails, normal activity in the cell can be disrupted, which triggers ill health and could be a factor in diseases such as cancer and neurodegeneration. To find cures for major illnesses, scientists need to understand the complex shape of DNA tangles. Existing lab techniques enable them to plot the shape and structure of DNA tangles but it is laborious and time-consuming. An international scientific team led by the University of Sheffield in the UK has now automated the process. Using what is known as an atomic force microscope, advanced computer software and AI - they are able to visualize the DNA molecules, trace their paths and measure them. Understanding the way DNA changes shape, a field of science known as DNA topology, requires researchers to conduct analysis at the nanoscale, where a nanometre is one billionth of a metre. Alice Pyne, Professor of Biophysics at the University of Sheffield, who supervised the research, said: "This is the first time we have been able to determine the structure of individual complex DNA structures found in cells with nanometre precision. We have done that by developing advanced new image analysis tools that can do in a matter of seconds that before may have taken hours. "This will allow us to look at what complex structures may be formed in the cell during normal and abnormal cellular processes, such as DNA replication and understand their implications. From here, we can start to look at how these complex topologies and structures affect proteins interacting with the genome, for example key antibiotic and anti-cancer targets such as topoisomerases (an enzyme that untangles knotted DNA)." Dr Sean Colloms, from the School of Molecular Bioscience at the University of Glasgow and a co-author of the study, said: "DNA is a really long molecule. Just like any long piece of string, the DNA in our cells gets tangled and knotted. If we want to study the processes in cells that lead to DNA knotting, as well as the action of topoisomerases to remove the knotting, we need to be able to determine exactly how the DNA is tangled. "At each DNA crossing, we can see which piece of DNA goes over which and this even allows us to tell the difference between one knot and its mirror image, which is important in our studies." An atomic force microscope uses a tiny probe to physically measure the object under analysis - rather than light or electrons as in other types of microscope. That difference makes it suitable for nanoscale analysis. "Molecular simulations help us understand how DNA interacts with mica surfaces in AFM experiments," said Dušan Račko from the Polymer Institute of the Slovak Academy of Sciences, who was involved in the study. "By developing advanced models, we can generate thousands of molecular structures to train future AI frameworks - bringing us closer to visualizing and understanding topology of complex DNA assemblies." The study is the culmination of an international research collaboration involving scientists from 6 universities and research institutes from across the UK, Slovakia and France. University of Sheffield Journal reference: Holmes, E. P., et al. (2025). Quantifying complexity in DNA structures with high resolution Atomic Force Microscopy. Nature Communications. doi.org/10.1038/s41467-025-60559-x.
[3]
AI tool untangles DNA knots to help predict health impacts - Earth.com
DNA bends, loops, and crosses itself in cramped cells, and those 'knots' can help or hinder life. Scientists have long taught the neat ladder-like twist, but spent years wrestling with the messier truth. Researchers have now developed a rapid method to visualize DNA crossings and determine, at the level of single molecules, which strand passes over or under the other. The method pairs high-resolution imaging with smart software and quickly delivers answers that used to take a lot of time. The research was led by Alice Pyne, a professor of biophysics at the University of Sheffield. The group imaged DNA using atomic force microscopy (AFM) and then traced each molecule's path with a deep-learning pipeline that identifies every crossing and labels it as over or under. The team demonstrated that they can recover the topology, the length, and the local shape of single DNA circles and their tangles with sub-molecular detail. Unlike light-based microscopes, atomic force microscopy feels the surface with a nanoscale probe, which makes it well suited to single molecule work in fluid. A previous study showed that atomic force microscopy can map DNA, without staining, under near-cellular conditions. The Sheffield pipeline advances this by measuring the height profile at each strand crossing and applying a full-width-at-half-maximum comparison to identify which DNA strand passes over. This method increases accuracy, especially when crossings occur in close proximity. The software then outputs a topological class for the whole molecule, so researchers do not have to infer knot types by gel position or by eye. Cells rely on DNA topology to manage access to genes and to keep the genome intact. When the balance tips, damage builds up and repair systems struggle. A comprehensive review explains how each human topoisomerase family member cuts and rejoins DNA to manage supercoiling and catenation. It also shows how these enzymes serve as important targets for antibiotics and anti-cancer drugs. Those links are not abstract. Mismanaged crossings and twists during replication or transcription trigger breaks, stalled forks, altered gene expression, and increased disease risk. A method that cleanly reads which strand sits on top at each crossing can show when and where the cell's topological control slips, and it can reveal how drug candidates change those patterns. To show the software works beyond simple plasmids, the authors tested it on replication intermediates formed in Xenopus egg extracts. This well-established system mimics DNA synthesis outside a living cell. Protocols for these extracts show they contain all the factors needed to license and copy DNA, which lets scientists pause forks and capture real intermediates. They also created specific DNA knots and links using E. coli proteins and then tested whether the system could correctly identify them. The team demonstrated that the method could measure the size of simple DNA circles with about one percent accuracy. It also distinguished between two similar five-crossing knot types by tracking how the strands crossed over or under each other. "We have done that by developing advanced new image-analysis tools that can do in a matter of seconds that before may have taken hours," said Pyne. The hardest part of visualizing a tangle is not seeing that two segments cross - it is telling which one is on top. The authors solved this problem by using the small but measurable height difference at each crossing. The researchers then trained a model to follow the DNA paths without losing track at tight junctions. "DNA is a really long molecule," said study co-author Dr. Sean Colloms from the School of Molecular Bioscience at the University of Glasgow. "Just like any long piece of string, the DNA in our cells gets tangled and knotted." "At each DNA crossing, we can see which piece of DNA goes over which and this even allows us to tell the difference between one knot and its mirror image, which is important in our studies." The ability to classify tangles on single molecules gives drug hunters a readout for compounds that alter replication, transcription, or decatenation. It can also guide design in DNA nanotechnology, where loops and crossings are part of the scaffold rather than a nuisance. Previous studies have already used atomic force microscopy to see how supercoiling changes groove width and local recognition. This work suggests that shape cues may help guide protein binding. "By developing advanced models, we can generate thousands of molecular structures to train future AI frameworks, bringing us closer to visualizing and understanding topology of complex DNA assemblies," said Dušan Račko of the Polymer Institute of the Slovak Academy of Sciences (SAS). Every method has trade-offs. Atomic force microscopy requires attaching DNA to a surface and pushing gently with a tip, so adsorption and imaging forces can change conformation if conditions are not tuned well. A 2021 AFM review shows how cations, polymers, and scan settings shape mica results, stressing consistency. Even so, the Sheffield pipeline reduces observer bias and scales analysis so that tricky cases can be flagged by their confidence scores instead of quietly misread. It also makes replication biology more concrete by turning fork junctions, gaps, and reversed forks into numbers rather than sketches. That shift from impression to measurement is what lets the field ask sharper questions. There is room to grow. The same tracing approach should extend to RNA, to protein-nucleic acid complexes, and to engineered lattices. Adding live imaging and selective chemistries could link topological snapshots to time and to specific proteins, which could refine readouts for topoisomerase drugs and beyond. Like what you read? Subscribe to our newsletter for engaging articles, exclusive content, and the latest updates.
Share
Share
Copy Link
An international research team has developed an automated technique using AI and atomic force microscopy to visualize and measure complex DNA structures with nanometer precision, potentially advancing our understanding of DNA topology and its role in diseases.
Breakthrough in DNA Visualization
An international research team, led by the University of Sheffield, has developed a groundbreaking automated technique to visualize and measure complex DNA structures with unprecedented precision. This innovative approach combines atomic force microscopy (AFM), advanced computer software, and artificial intelligence to revolutionize our understanding of DNA topology
1
.
Source: Phys.org
The Complexity of DNA Structures
DNA, often depicted as a tidy double helix in textbooks, actually forms intricate and varied shapes within living cells. As the molecule is extremely long, it tends to twist and tangle upon itself. While enzymes in the body attempt to regulate this process, failures in regulation can disrupt normal cellular activity, potentially contributing to diseases such as cancer and neurodegeneration
2
.Advanced Imaging and AI Analysis
The new technique utilizes an atomic force microscope, which employs a tiny probe to physically measure objects at the nanoscale. This approach, combined with AI-powered image analysis, allows researchers to:
- Visualize DNA molecules
- Trace their paths
- Measure their structures
Professor Alice Pyne, who supervised the research, emphasized the significance of this development: "This is the first time we have been able to determine the structure of individual complex DNA structures found in cells with nanometer precision"
1
.Implications for Disease Research and Drug Development
Understanding DNA topology is crucial for developing treatments for major illnesses. The new method enables researchers to examine complex structures formed during normal and abnormal cellular processes, such as DNA replication. This knowledge could lead to insights into how these structures affect proteins interacting with the genome, including key antibiotic and anti-cancer targets like topoisomerases
2
.Molecular Simulations and AI Training

Source: Earth.com
Dr. Dušan Račko from the Polymer Institute of the Slovak Academy of Sciences highlighted the role of molecular simulations in understanding DNA interactions with mica surfaces in AFM experiments. These simulations generate thousands of molecular structures, which can be used to train future AI frameworks, bringing researchers closer to visualizing and understanding the topology of complex DNA assemblies
3
.Related Stories
Rapid Analysis and Increased Accuracy
The Sheffield pipeline significantly reduces analysis time, performing in seconds what previously took hours. It also improves accuracy by measuring the height profile at each strand crossing and applying a full-width-at-half-maximum comparison to identify which DNA strand passes over another. This method is particularly effective when crossings occur in close proximity
3
.Future Applications and Potential
While the current focus is on DNA, the same tracing approach could potentially extend to RNA, protein-nucleic acid complexes, and engineered lattices. Future developments may include live imaging and selective chemistries, linking topological snapshots to time and specific proteins. This could refine readouts for topoisomerase drugs and open up new avenues for research in molecular biology and medicine
3
.References
Summarized by
Navi
Related Stories
MIT Chemists Revolutionize 3D Genome Structure Prediction with Generative AI
01 Feb 2025•Science and Research

AI Breakthrough: Designing Synthetic DNA Switches for Precise Gene Control
24 Oct 2024•Science and Research

AI-Powered Method Unveils 'Hyperaccessible' DNA Window, Revolutionizing Genomic Research
22 Jan 2025•Science and Research

Recent Highlights
1
OpenAI Releases GPT-5.4, New AI Model Built for Agents and Professional Work
Technology

2
Anthropic sues Pentagon over supply chain risk label after refusing autonomous weapons use
Policy and Regulation

3
OpenAI secures $110 billion funding round as questions swirl around AI bubble and profitability
Business and Economy