AI-Powered 'Pythia' Tool Revolutionizes Precision in CRISPR Gene Editing
3 Sources
3 Sources
[1]
AI meets CRISPR for precise gene editing
A research team headed by the University of Zurich has developed a powerful new method to precisely edit DNA by combining cutting-edge genetic engineering with artificial intelligence. The work has been published in Nature Biotechnology. This technique opens the door to more accurate modeling of human diseases and lays the groundwork for next-generation gene therapies. Precise and targeted DNA editing by small point mutations as well as the integration of whole genes via CRISPR/Cas technology has great potential for applications in biotechnology and gene therapy. However, it is very important that the so-called "gene scissors" do not cause any unintended genetic changes, but maintain genomic integrity to avoid unintended side effects. Normally, double-stranded breaks in the DNA molecule are accurately repaired in humans and other organisms. But occasionally, this DNA end-joining repair results in genetic errors. Gene editing with greatly improved precision Now, scientists from the University of Zurich (UZH), Ghent University in Belgium and the ETH Zurich have developed a new method which greatly improves the precision of genome editing. Using artificial intelligence (AI), the tool called "Pythia" predicts how cells repair their DNA after it is cut by gene-editing tools such as CRISPR/Cas9. "Our team developed tiny DNA repair templates, which act like molecular glue and guide the cell to make precise genetic changes," says lead author Thomas Naert, who pioneered the technology at the UZH and is currently a post-doc at Gent University. These AI-designed templates were first tested in human cell cultures, where they enabled highly accurate gene edits and integrations. The approach was also validated in other organisms, including Xenopus, a small tropical frog used in biomedical research, and in living mice, where the researchers successfully edited DNA in brain cells. AI can learn and predict DNA repair patterns "DNA repair follows patterns; it is not random. And Pythia uses these patterns to our advantage," says Naert. Traditionally, when CRISPR cuts DNA, scientists rely on the cell's natural repair mechanisms to fix the break. While these repairs follow predictable patterns, they can result in unwanted outcomes, such as destruction of the surrounding genes. "What we modeled at a massive scale is that this DNA repair process obeys consistent rules that AI can learn and predict," says Naert. With this insight, the researchers simulated millions of possible editing outcomes using machine learning, asking a simple but powerful question: What is the most efficient way to make a specific small change to the genome, given how the cell is likely to repair itself? In addition to changing individual letters of the genetic code or integrating an exogenously delivered gene, the method can also be used to fluorescently label specific proteins. "That is incredibly powerful," says Naert, "because it allows us to directly observe what individual proteins are doing in healthy and diseased tissue." Another advantage of the new method is that it works well in all cells -- even in organs with no cell division, such as the brain. Basis for developing precise gene therapies Pythia is named after the high priestess of the oracle at the Temple of Apollo of Delphi in Antiquity, who was consulted to predict the future. In a similar way, this new tool allows scientists to forecast the outcomes of gene editing with remarkable precision. "Just as meteorologists use AI to predict the weather, we are using it to forecast how cells will respond to genetic interventions. That kind of predictive power is essential if we want gene editing to be safe, reliable, and clinically useful," says Soeren Lienkamp, professor at the Institute of Anatomy of UZH and the ETH Zurich and senior author of the study. "What excites us most is not only the technology itself, but also the possibilities it opens. Pythia brings together large-scale AI prediction with real biological systems. From cultured cells to whole animals, this tight loop between modeling and experimentation points is becoming increasingly useful, for example in precise gene therapies," Lienkamp adds. This work creates new possibilities for understanding genetic disease and developing gene therapies, also for neurological diseases, that are both safer and more effective.
[2]
New method greatly improves the precision of genome editing
University of ZurichAug 12 2025 A research team headed by the University of Zurich has developed a powerful new method to precisely edit DNA by combining cutting-edge genetic engineering with artificial intelligence. This technique opens the door to more accurate modeling of human diseases and lays the groundwork for next-generation gene therapies. Precise and targeted DNA editing by small point mutations as well as the integration of whole genes via CRISPR/Cas technology has great potential for applications in biotechnology and gene therapy. However, it is very important that the so-called "gene scissors" do not cause any unintended genetic changes, but maintain genomic integrity to avoid unintended side effects. Normally, double-stranded breaks in the DNA molecule are accurately repaired in humans and other organisms. But occasionally, this DNA end joining repair results in genetic errors. Gene editing with greatly improved precision Now, scientists from the University of Zurich (UZH), Ghent University in Belgium and the ETH Zurich have developed a new method which greatly improves the precision of genome editing. Using artificial intelligence (AI), the tool called "Pythia" predicts how cells repair their DNA after it is cut by gene editing tools such as CRISPR/Cas9. "Our team developed tiny DNA repair templates, which act like molecular glue and guide the cell to make precise genetic changes", says lead author Thomas Naert, who pioneered the technology in at the UZH and is currently a post-doc at Gent University. These AI-designed templates were first tested in human cell cultures, where they enabled highly accurate gene edits and integrations. The approach was also validated in other organisms, including Xenopus, a small tropical frog used in biomedical research, and in living mice, where the researchers successfully edited DNA in brain cells. AI can learn and predict DNA repair patterns "DNA repair follows patterns; it is not random. And Pythia uses these patterns to our advantage," says Naert. Traditionally, when CRISPR cuts DNA, scientists rely on the cell's natural repair mechanisms to fix the break. While these repairs follow predictable patterns, they can result in unwanted outcomes, such as destruction of the surrounding genes. What we modeled at massive scale is that this DNA repair process obeys consistent rules that AI can learn and predict." Thomas Naert, lead author With this insight, the researchers simulated millions of possible editing outcomes using machine learning, asking a simple but powerful question: What is the most efficient way to make a specific small change to the genome, given how the cell is likely to repair itself? In addition to changing individual letters of the genetic code or integrate an exogenously delivered gene, the method can also be used to fluorescently label specific proteins. "That is incredibly powerful," says Naert, "because it allows us to directly observe what individual proteins are doing in healthy and diseased tissue." Another advantage of the new method is that it works well in all cells - even in organs with no cell division, such as the brain. Basis for developing precise gene therapies Pythia is named after the high priestess of the oracle at the Temple of Apollo of Delphi in Antiquity, who was consulted to predict the future. In a similar way, this new tool allows scientists to forecast the outcomes of gene editing with remarkable precision. "Just as meteorologists use AI to predict the weather, we are using it to forecast how cells will respond to genetic interventions. That kind of predictive power is essential if we want gene editing to be safe, reliable, and clinically useful," says Soeren Lienkamp, professor at the Institute of Anatomy of UZH and the ETH Zurich and senior author of the study. "What excites us most is not only the technology itself, but also the possibilities it opens. Pythia brings together large-scale AI prediction with real biological systems. From cultured cells to whole animals, this tight loop between modeling and experimentation points is becoming increasingly useful, for example in precise gene therapies", Lienkamp adds. This work creates new possibilities for understanding genetic disease and developing gene therapies, also for neurological diseases, that are both safer and more effective. University of Zurich Journal reference: Naert, T., et al. (2025) Precise, predictable genome integrations by deep-learning-assisted design of microhomology-based templates. Nature Biotechnology. doi.org/10.1038/s41587-025-02771-0.
[3]
Scientists Are Using AI for Improved Gene Hacking
Gene editing has made huge leaps in recent years, such as treating the congenital blood disorders sickle cell anemia and beta thalassemia, which can require lifelong blood transfusions. But scientists still fear that some snipping may lead to unwelcome surprises. However, a research team led by the University of Zurich says that artificial intelligence could help. A new study published in the journal Nature details how the researchers combined AI and the gene editing technique CRISPR-Cas for more precise editing, which may lead to better gene therapies for patients and fewer side effects. They did this by developing a new AI tool called Pythia, named after the ancient oracle at the Temple of Apollo, which can predict how cells will repair a portion of DNA post-gene editing. "DNA repair follows patterns; it's not random," said lead author and University of Zurich biotechnology researcher Thomas Naert in a statement about the study. "We were able to model on a large scale that this DNA repair process obeys consistent rules that AI can learn and predict." To test out the efficacy of Pythia, the scientists developed what they called "DNA repair templates" based on Pythia's predictions. "Our team has developed tiny DNA repair templates that act like molecular glue, guiding the cell to make precise genetic changes," said Naert. They were successful in making precise repairs on human cell cultures and then on lab mice and small frogs, which are often used for science experiments. Scientists observed the altered cells that were subjected to a DNA repair template and saw that edits didn't negatively impact the process of cell division or the subsequent functions within the cells. Their experiments with Pythia could enable scientists to precisely change a few letters or insert strings of genetic code into their correct location, while also having the ability to work with cells that don't divide or replicate like mature brain cells. Besides more accurate gene editing, Pythia could also allow researchers to precisely tag genes that make certain proteins with fluorescent labels that glow under microscopic observation. "This allows us to directly observe what individual proteins do in healthy and diseased tissue," said Naert. The scientists are hopeful that others could make use of their AI model, potentially inspiring more treatments for intractable diseases caused by faulty genes.
Share
Share
Copy Link
Researchers from the University of Zurich have developed an AI tool called Pythia that significantly improves the precision of CRISPR gene editing, opening new possibilities for disease modeling and gene therapies.
AI Meets CRISPR: A Breakthrough in Precision Gene Editing
Researchers from the University of Zurich, in collaboration with Ghent University and ETH Zurich, have developed a groundbreaking method that combines artificial intelligence (AI) with CRISPR gene editing technology. This innovative approach, detailed in a study published in Nature Biotechnology, promises to revolutionize the field of genetic engineering by significantly improving the precision of genome editing
1
.
Source: News-Medical
Introducing Pythia: AI-Powered DNA Repair Prediction
At the heart of this advancement is a new AI tool called "Pythia," named after the ancient Greek oracle. Pythia's primary function is to predict how cells will repair their DNA after it has been cut by gene-editing tools like CRISPR/Cas9
2
.Lead author Thomas Naert explains, "DNA repair follows patterns; it is not random. And Pythia uses these patterns to our advantage." By leveraging machine learning to simulate millions of possible editing outcomes, Pythia can determine the most efficient way to make specific genetic changes while considering the cell's likely repair mechanisms
1
.Tiny Templates for Precise Genetic Changes
The research team developed small DNA repair templates based on Pythia's predictions. These templates act as "molecular glue," guiding cells to make precise genetic alterations. This approach has been successfully tested in human cell cultures, Xenopus frogs (commonly used in biomedical research), and living mice, where researchers even managed to edit DNA in brain cells
3
.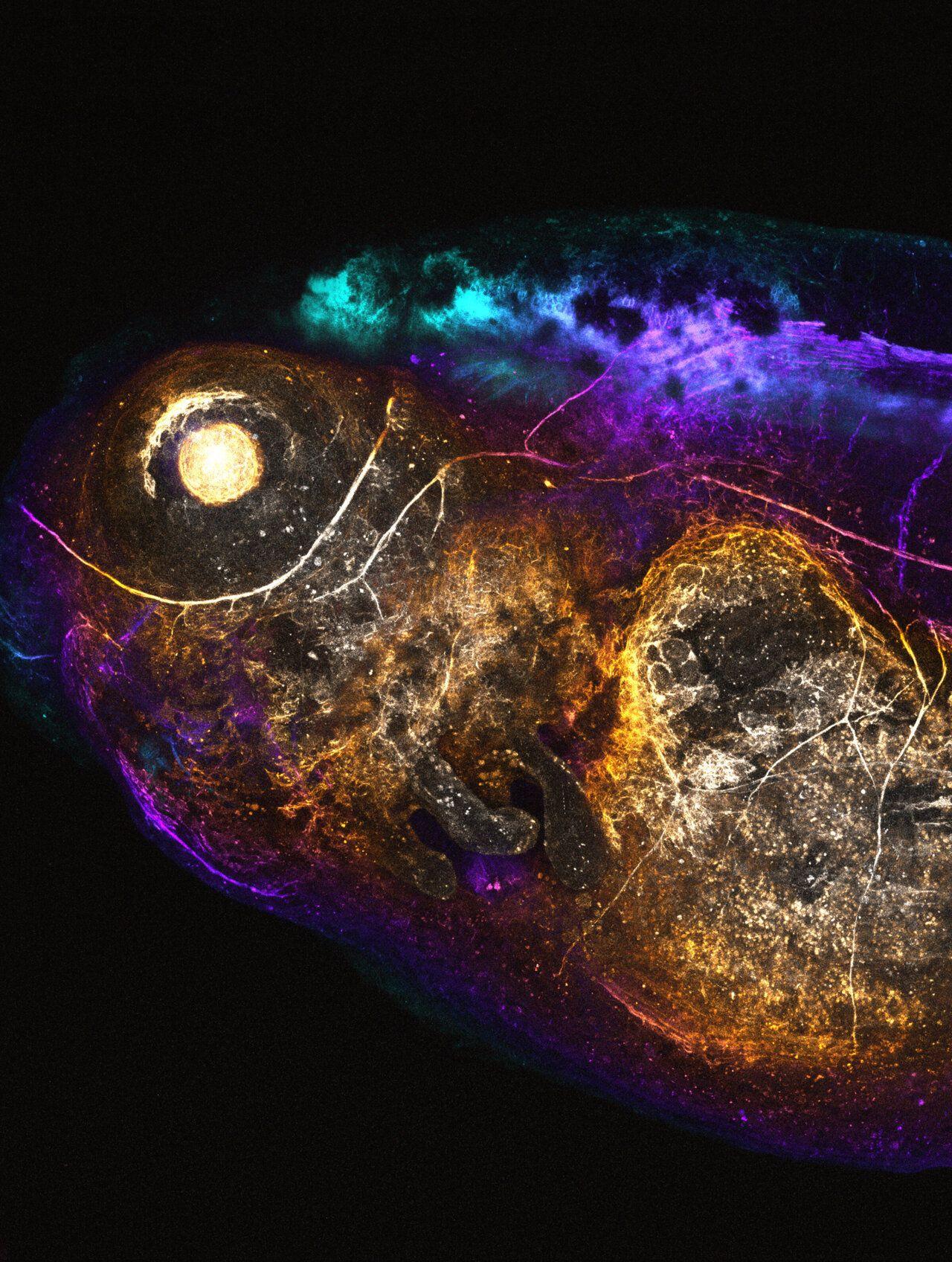
Source: Phys.org
Versatility and Power of the New Method
The Pythia-assisted method offers several key advantages:
- Precision: It allows for highly accurate gene edits and integrations, reducing the risk of unintended genetic changes.
- Versatility: The technique can change individual letters of the genetic code, integrate whole genes, and even fluorescently label specific proteins for observation.
- Wide applicability: It works effectively in all cells, including those in organs with no cell division, such as the brain
2
.
Related Stories
Implications for Disease Modeling and Gene Therapy
Professor Soeren Lienkamp, senior author of the study, emphasizes the potential of this technology: "Pythia brings together large-scale AI prediction with real biological systems. From cultured cells to whole animals, this tight loop between modeling and experimentation points is becoming increasingly useful, for example in precise gene therapies"
1
.This breakthrough creates new possibilities for understanding genetic diseases and developing safer, more effective gene therapies, including those for neurological conditions. The ability to observe protein behavior in healthy and diseased tissues through fluorescent labeling adds another powerful dimension to this tool
3
.
Source: Futurism
Future Prospects and Challenges
While the potential of Pythia is immense, researchers acknowledge that further validation and refinement will be necessary before its widespread application in clinical settings. However, the successful integration of AI and gene editing technologies marks a significant step forward in the field of genetic engineering, potentially accelerating the development of personalized medicine and targeted therapies for a wide range of genetic disorders.
References
Summarized by
Navi
[1]
[2]
Related Stories
CRISPR-GPT: AI-Powered Gene Editing Assistant Accelerates Drug Development
17 Sept 2025•Science and Research

AI Breakthrough: Designing Synthetic DNA Switches for Precise Gene Control
24 Oct 2024•Science and Research

AI Designs DNA to Control Gene Expression in Healthy Mammalian Cells
09 May 2025•Science and Research

Recent Highlights
1
Google Gemini 3.1 Pro doubles reasoning score, beats rivals in key AI benchmarks
Technology

2
Pentagon Summons Anthropic CEO as $200M Contract Faces Supply Chain Risk Over AI Restrictions
Policy and Regulation

3
Canada Summons OpenAI Executives After ChatGPT User Became Mass Shooting Suspect
Policy and Regulation