AI-Powered Tool DECIPHAER Unveils Molecular Mechanisms of Tuberculosis Drug Action
3 Sources
3 Sources
[1]
AI-powered analysis reveals how drugs kill tuberculosis at the molecular level
Tuberculosis (TB) is the world's deadliest infectious disease -- and one of the hardest to cure. Standard treatment requires a cocktail of multiple drugs over at least six months, and one in five patients have a type of TB that resists these first-line medications. Now, a new study offers a powerful AI-assisted method for uncovering exactly how TB drugs kill the bacteria, opening the door to smarter treatment combinations that could work faster. Developing a more effective -- and shorter -- TB treatment is a global priority. "We need a better multidrug regimen: three to five new drugs that work even for what is currently drug-resistant TB," says Bree Aldridge, senior author of the study and a professor in molecular biology and microbiology at Tufts University School of Medicine and professor in biomedical engineering at Tufts University School of Engineering. But progress has been slow, in part, because scientists lacked tools to see precisely how drugs work and therefore how they could best work together to attack TB bacteria. "TB likely has multiple Achilles' heels that we could hit all at once," explains Aldridge, who also is associate director of the Stuart B. Levy Center for Integrated Management of Antimicrobial Resistance at Tufts. "But it's surprisingly difficult to figure out exactly how a drug kills its target cell." It's like walking into a room and spying bruised faces, an overturned chair, and a shattered lamp; you can tell that a fight happened but not who started it or how it unfolded. In the same way, scientists can tell when a drug has killed target cells, but often not the exact chain of molecular events, aka, "mechanism of death." Aldridge and her collaborators from Tufts University School of Medicine and other institutions have now found a way to understand that mechanism. In a new study in Cell Systems, they demonstrated how their new AI-assisted tool -- called DECIPHAER (decoding cross-modal information of pharmacologies via autoencoders) -- can reveal, in molecular detail, how potential TB drugs kill the bacteria. The tool builds on the team's earlier research that captured high-resolution images of TB bacteria as they die during treatment. These snapshots reveal clues -- for example, changes in the bacterial cells' shape or internal structure -- caused by a drug's mode of attack. Scientists use this "morphological profiling" as a kind of crime scene investigation for cells: They dose TB bacteria with a new drug, freeze them at the moment of death, and compare the resulting cellular damage with patterns seen from known antibiotics. "If you treat TB bacteria with a new drug and it goes splat in the same way it does for other drugs that destroy the cell wall, then you may assume it destroys the cell wall as well," says Aldridge. Using AI, the team has now gone a step further, linking these visual clues to detailed readouts of bacterial gene activity, known as transcriptional profiles. The researchers trained a model to spot which molecular changes, such as bacterial genes switching on or off, occur alongside specific visual changes. "Before, we could only say roughly how a drug killed TB using morphological profiling. Now we can bring more exact insights into how drugs are impacting the cells and why the bacteria are dying," says Aldridge. For example, in testing DECIPHAER, she says the team found that a TB drug in clinical development didn't work as expected. "Based on similar existing compounds, we had assumed the drug worked by destroying the cell wall," she says. "But it actually kills TB bacteria by impairing the respiratory chain and cells' ability to make energy." Because the AI tool can predict a drug's molecular impact from images alone -- which is far cheaper than using RNA sequencing -- it can faster reveal how potential TB treatments work in different growth conditions, genetic strains, or drug combinations. "We plan to keep using it in our own lab's drug combination studies and hope it will support collaborations worldwide to accelerate the development of new TB drugs," says Aldridge. While the need is especially urgent for TB, she adds that DECIPHAER's approach also could be applied to other infectious diseases and cancer.
[2]
AI tool reveals how TB drugs kill bacteria at the molecular level
Tufts UniversityAug 25 2025 Tuberculosis (TB) is the world's deadliest infectious disease -- and one of the hardest to cure. Standard treatment requires a cocktail of multiple drugs over at least six months, and one in five patients have a type of TB that resists these first-line medications. Now, a new study offers a powerful AI-assisted method for uncovering exactly how TB drugs kill the bacteria, opening the door to smarter treatment combinations that could work faster. Developing a more effective -- and shorter -- TB treatment is a global priority. "We need a better multidrug regimen: three to five new drugs that work even for what is currently drug-resistant TB," says Bree Aldridge, senior author of the study and a professor in molecular biology and microbiology at Tufts University School of Medicine and professor in biomedical engineering at Tufts University School of Engineering. But progress has been slow, in part, because scientists lacked tools to see precisely how drugs work and therefore how they could best work together to attack TB bacteria. "TB likely has multiple Achilles' heels that we could hit all at once," explains Aldridge, who also is associate director of the Stuart B. Levy Center for Integrated Management of Antimicrobial Resistance at Tufts. "But it's surprisingly difficult to figure out exactly how a drug kills its target cell." It's like walking into a room and spying bruised faces, an overturned chair, and a shattered lamp; you can tell that a fight happened but not who started it or how it unfolded. In the same way, scientists can tell when a drug has killed target cells but often not the exact chain of molecular events, aka, "mechanism of death." Aldridge and her collaborators from Tufts University School of Medicine and other institutions now have found a way to understand that mechanism. In a new study in Cell Systems, they demonstrated how their new AI-assisted tool -- called DECIPHAER (decoding cross-modal information of pharmacologies via autoencoders) -- can reveal, in molecular detail, how potential TB drugs kill the bacteria. The tool builds on the team's earlier research that captured high-resolution images of TB bacteria as they die during treatment. These snapshots reveal clues -- for example, changes in the bacterial cells' shape or internal structure -- caused by a drug's mode of attack. Scientists use this "morphological profiling" as a kind of crime scene investigation for cells: They dose TB bacteria with a new drug, freeze them at the moment of death, and compare the resulting cellular damage with patterns seen from known antibiotics. If you treat TB bacteria with a new drug and it goes splat in the same way it does for other drugs that destroy the cell wall, then you may assume it destroys the cell wall as well." Bree Aldridge, senior author of the study Using AI, the team now has gone a step further, linking these visual clues to detailed readouts of bacterial gene activity, known as transcriptional profiles. The researchers trained a model to spot which molecular changes, such as bacterial genes switching on or off, occur alongside specific visual changes. "Before, we could only say roughly how a drug killed TB using morphological profiling. Now we can bring more exact insights into how drugs are impacting the cells and why the bacteria are dying," says Aldridge. For example, in testing DECIPHAER, she says the team found that a TB drug in clinical development didn't work as expected. "Based on similar existing compounds, we had assumed the drug worked by destroying the cell wall," she says. "But it actually kills TB bacteria by impairing the respiratory chain and cells' ability to make energy." Because the AI tool can predict a drug's molecular impact from images alone -- which is far cheaper than using RNA sequencing -- it can faster reveal how potential TB treatments work in different growth conditions, genetic strains, or drug combinations. "We plan to keep using it in our own lab's drug combination studies and hope it will support collaborations worldwide to accelerate development of new TB drugs," says Aldridge. While the need is especially urgent for TB, she adds that DECIPHAER's approach also could be applied to other infectious diseases and cancer. William C. Johnson, a Ph.D. student in molecular microbiology at Tufts Graduate School of Biomedical Sciences, is the first author. Research reported in this article was supported in part by the Gates Foundation and by the National Institutes of Health under award number T32AI007422. Complete information on authors, funders, methodology, limitations, and conflicts of interest is available in the published paper. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders. Tufts University Journal reference: Johnson, W. C., et al. (2025). Integration of multi-modal measurements identifies critical mechanisms of tuberculosis drug action. Cell Systems. doi.org/10.1016/j.cels.2025.101348
[3]
New AI Tool Reveals How Drugs Kill Tuberculosis | Newswise
Newswise -- Tuberculosis (TB) is the world's deadliest infectious disease -- and one of the hardest to cure. Standard treatment requires a cocktail of multiple drugs over at least six months, and one in five patients have a type of TB that resists these first-line medications. Now, a new study offers a powerful AI-assisted method for uncovering exactly how TB drugs kill the bacteria, opening the door to smarter treatment combinations that could work faster. Developing a more effective -- and shorter -- TB treatment is a global priority. "We need a better multidrug regimen: three to five new drugs that work even for what is currently drug-resistant TB," says Bree Aldridge, senior author of the study and a professor in molecular biology and microbiology at Tufts University School of Medicine and professor in biomedical engineering at Tufts University School of Engineering. But progress has been slow, in part, because scientists lacked tools to see precisely how drugs work and therefore how they could best work together to attack TB bacteria. "TB likely has multiple Achilles' heels that we could hit all at once," explains Aldridge, who also is associate director of the Stuart B. Levy Center for Integrated Management of Antimicrobial Resistance at Tufts. "But it's surprisingly difficult to figure out exactly how a drug kills its target cell." It's like walking into a room and spying bruised faces, an overturned chair, and a shattered lamp; you can tell that a fight happened but not who started it or how it unfolded. In the same way, scientists can tell when a drug has killed target cells but often not the exact chain of molecular events, aka, "mechanism of death." Aldridge and her collaborators from Tufts University School of Medicine and other institutions now have found a way to understand that mechanism. In a new study in Cell Systems, they demonstrated how their new AI-assisted tool -- called DECIPHAER (decoding cross-modal information of pharmacologies via autoencoders) -- can reveal, in molecular detail, how potential TB drugs kill the bacteria. The tool builds on the team's earlier research that captured high-resolution images of TB bacteria as they die during treatment. These snapshots reveal clues -- for example, changes in the bacterial cells' shape or internal structure -- caused by a drug's mode of attack. Scientists use this "morphological profiling" as a kind of crime scene investigation for cells: They dose TB bacteria with a new drug, freeze them at the moment of death, and compare the resulting cellular damage with patterns seen from known antibiotics. "If you treat TB bacteria with a new drug and it goes splat in the same way it does for other drugs that destroy the cell wall, then you may assume it destroys the cell wall as well," says Aldridge. Using AI, the team now has gone a step further, linking these visual clues to detailed readouts of bacterial gene activity, known as transcriptional profiles. The researchers trained a model to spot which molecular changes, such as bacterial genes switching on or off, occur alongside specific visual changes. "Before, we could only say roughly how a drug killed TB using morphological profiling. Now we can bring more exact insights into how drugs are impacting the cells and why the bacteria are dying," says Aldridge. For example, in testing DECIPHAER, she says the team found that a TB drug in clinical development didn't work as expected. "Based on similar existing compounds, we had assumed the drug worked by destroying the cell wall," she says. "But it actually kills TB bacteria by impairing the respiratory chain and cells' ability to make energy." Because the AI tool can predict a drug's molecular impact from images alone -- which is far cheaper than using RNA sequencing -- it can faster reveal how potential TB treatments work in different growth conditions, genetic strains, or drug combinations. "We plan to keep using it in our own lab's drug combination studies and hope it will support collaborations worldwide to accelerate development of new TB drugs," says Aldridge. While the need is especially urgent for TB, she adds that DECIPHAER's approach also could be applied to other infectious diseases and cancer. William C. Johnson, a Ph.D. student in molecular microbiology at Tufts Graduate School of Biomedical Sciences, is the first author. Research reported in this article was supported in part by the Gates Foundation and by the National Institutes of Health under award number T32AI007422. Complete information on authors, funders, methodology, limitations, and conflicts of interest is available in the published paper. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
Share
Share
Copy Link
Researchers at Tufts University have developed an AI-assisted tool called DECIPHAER that reveals how drugs kill tuberculosis bacteria at the molecular level, potentially accelerating the development of more effective treatments.
Breakthrough in Tuberculosis Research: AI-Powered Analysis Reveals Drug Mechanisms
Researchers at Tufts University have developed a groundbreaking AI-assisted tool that could revolutionize the treatment of tuberculosis (TB), the world's deadliest infectious disease. The tool, named DECIPHAER (decoding cross-modal information of pharmacologies via autoencoders), offers unprecedented insights into how drugs kill TB bacteria at the molecular level
1
2
3
.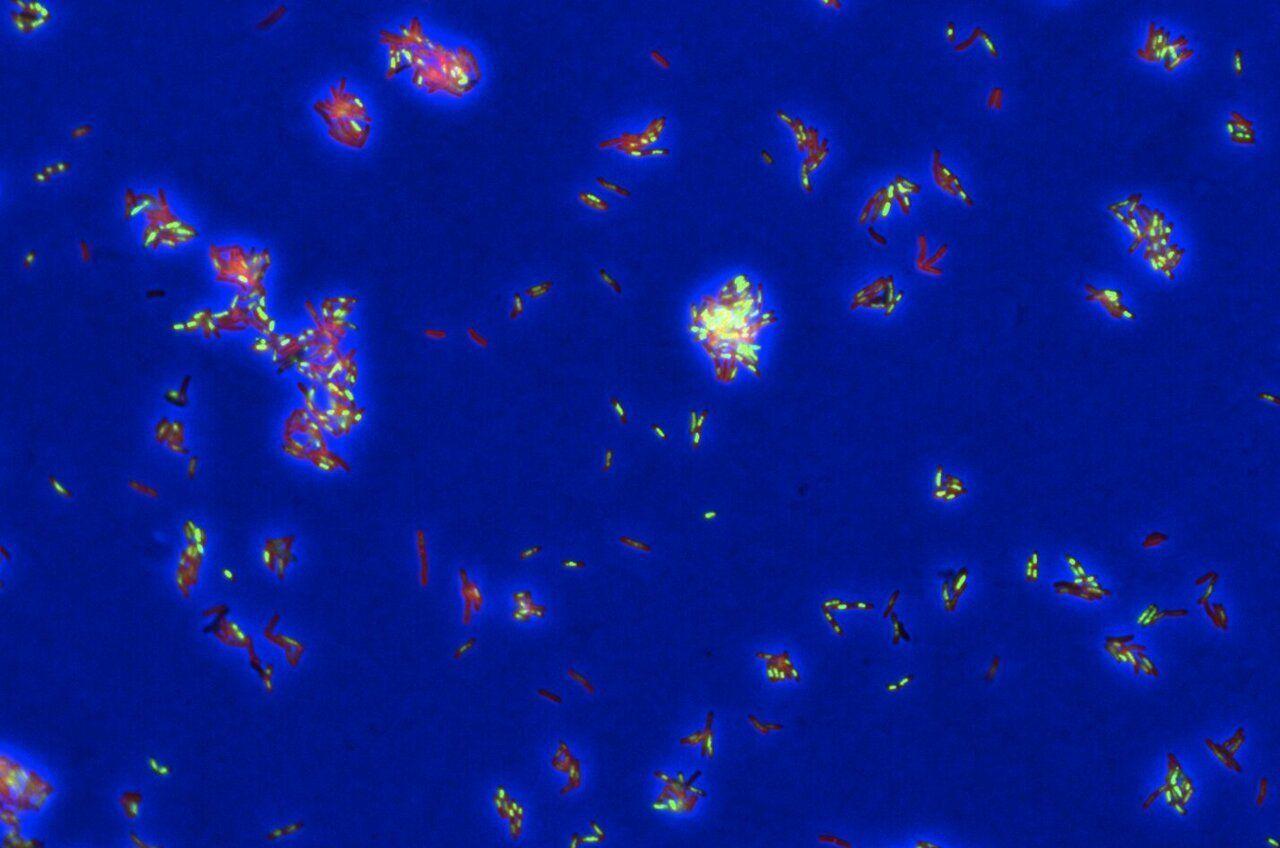
Source: Phys.org
The Challenge of Tuberculosis Treatment
TB remains a formidable global health challenge, with standard treatment requiring a cocktail of multiple drugs over at least six months. Moreover, one in five patients have drug-resistant TB, further complicating treatment efforts. Dr. Bree Aldridge, senior author of the study and professor at Tufts University, emphasizes the urgent need for a more effective multidrug regimen that can combat even drug-resistant TB
1
.DECIPHAER: Unraveling the Mystery of Drug Action
DECIPHAER builds upon previous research that captured high-resolution images of TB bacteria during treatment. The tool uses AI to link visual clues from these images to detailed readouts of bacterial gene activity, known as transcriptional profiles
2
. This innovative approach allows scientists to understand the exact chain of molecular events that occur when a drug kills TB bacteria.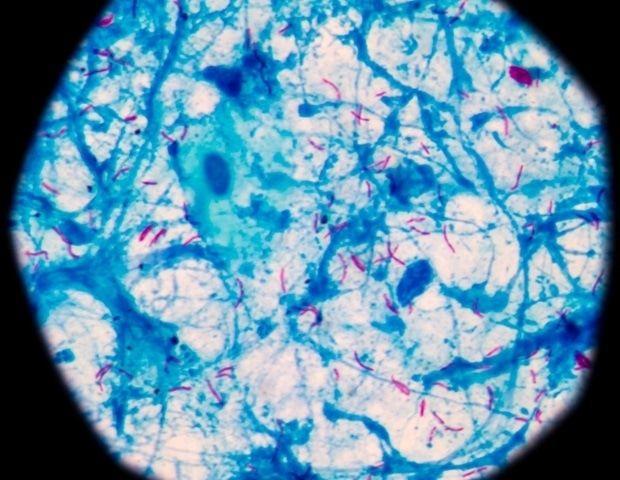
Source: News-Medical
From Visual Clues to Molecular Insights
The research team, led by Dr. Aldridge, trained an AI model to identify specific molecular changes that correspond to visual changes in the bacteria. This "morphological profiling" acts as a kind of cellular crime scene investigation, revealing how different drugs impact TB bacteria
3
.Surprising Discoveries and Future Applications
In testing DECIPHAER, the team made an unexpected discovery about a TB drug in clinical development. Dr. Aldridge explains, "Based on similar existing compounds, we had assumed the drug worked by destroying the cell wall. But it actually kills TB bacteria by impairing the respiratory chain and cells' ability to make energy"
1
2
.Related Stories
Accelerating Drug Development and Expanding Horizons
DECIPHAER's ability to predict a drug's molecular impact from images alone offers a cost-effective alternative to RNA sequencing. This could significantly speed up the process of understanding how potential TB treatments work in various conditions and combinations
3
.The researchers plan to continue using DECIPHAER in their drug combination studies and hope it will support global collaborations to accelerate the development of new TB drugs. While the immediate focus is on TB, Dr. Aldridge suggests that this approach could also be applied to other infectious diseases and cancer
1
2
3
.Funding and Future Prospects
The research, with William C. Johnson as the first author, was supported in part by the Gates Foundation and the National Institutes of Health. As the world continues to grapple with the challenge of TB and other infectious diseases, DECIPHAER represents a significant step forward in our ability to develop more effective treatments and combat drug resistance
2
3
.References
Summarized by
Navi
Related Stories
AI Breakthrough Accelerates Discovery of New Tuberculosis Drug Candidates
08 Feb 2025•Science and Research

AI Model Predicts Antibiotic Resistance in Bacteria with High Accuracy
03 Apr 2025•Science and Research
AI Accelerates Antibiotic Discovery: New Drug Targets IBD with Unprecedented Precision
03 Oct 2025•Health
Recent Highlights
1
Pentagon threatens to cut Anthropic's $200M contract over AI safety restrictions in military ops
Policy and Regulation

2
ByteDance's Seedance 2.0 AI video generator triggers copyright infringement battle with Hollywood
Policy and Regulation

3
OpenAI closes in on $100 billion funding round with $850 billion valuation as spending plans shift
Business and Economy





