Breakthrough in Lipid Imaging: New Method Reveals Rapid Protein-Mediated Lipid Transport in Cells
2 Sources
2 Sources
[1]
Quantitative imaging method reveals how cells rapidly sort and transport lipids
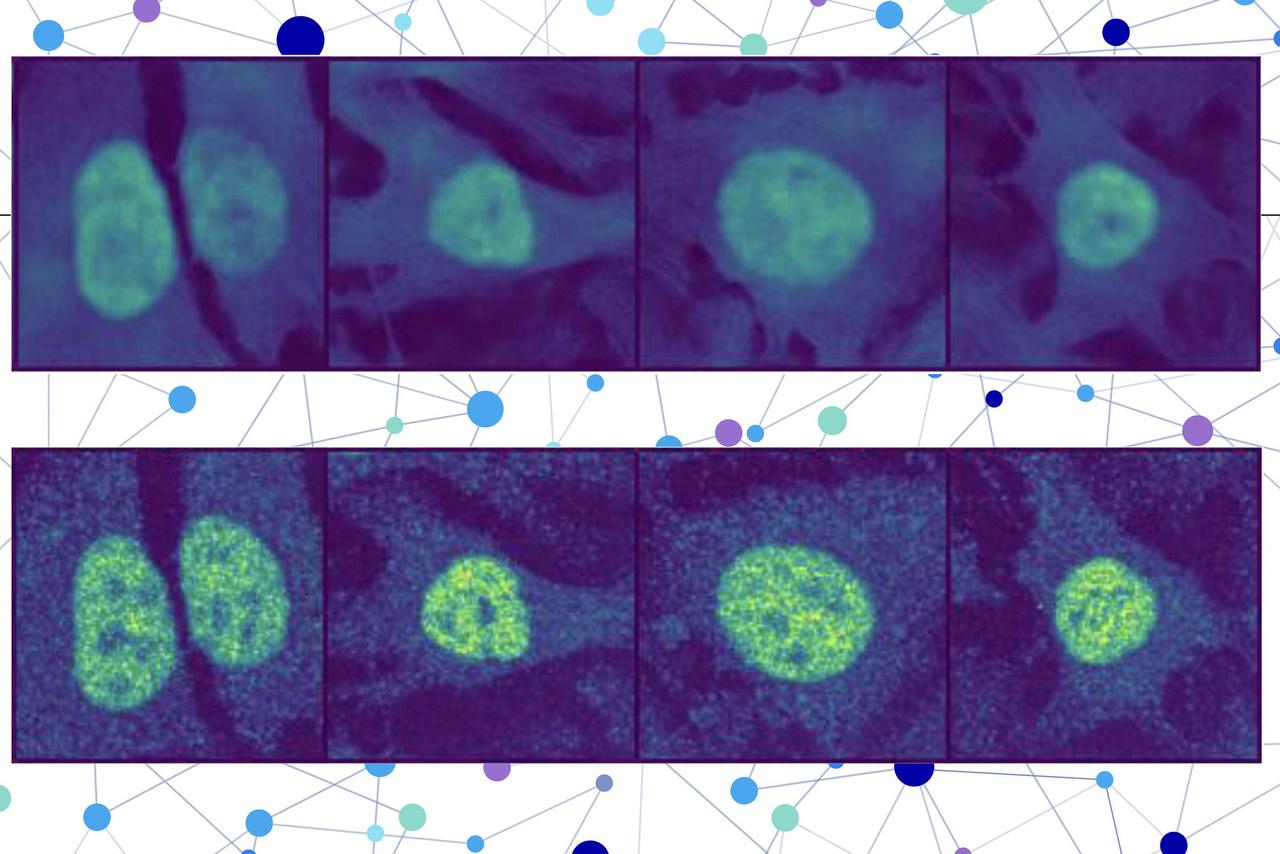
Lipids are difficult to detect with light microscopy. Using a new chemical labeling strategy, a Dresden-based team led by André Nadler at the Max Planck Institute of Molecular Cell Biology and Genetics (MPI-CBG) and Alf Honigmann at the Biotechnology Center (BIOTEC) of TU Dresden has overcome this limitation, enabling new insights into lipids. The researchers were able to answer a long-standing question: how do cells transport specific lipids to the membranes of their target organelles? The new lipid-imaging technique will help understand the role of lipid transport in health and disease. The findings were published in the journal Nature. Lipid molecules, or fats, are crucial to all forms of life. Cells need lipids to build membranes, separate and organize biochemical reactions, store energy, and transmit information. Every cell can create thousands of different lipids, and when they are out of balance, metabolic and neurodegenerative diseases can arise. It is still not well understood how cells sort different types of lipids between cell organelles to maintain the composition of each membrane. A major reason is that lipids are difficult to study, since microscopy techniques to precisely trace their location inside cells have so far been missing. In a long-standing collaboration, Nadler, a chemical biologist at the Max Planck Institute of Molecular Cell Biology and Genetics (MPI-CBG) in Dresden, Germany, teamed up with Honigmann, a bioimaging specialist at Biotechnology Center (BIOTEC) at the TU-Dresden University of Technology, to develop a method that enables visualizing lipids in cells using standard fluorescence microscopy. After the first successful proof of concept, the duo brought mass-spectrometry expert Andrej Shevchenko (MPI-CBG), Björn Drobot at the Helmholtz-Zentrum Dresden-Rossendorf (HZDR), and the group of Martin Hof from the J. Heyrovsky Institute of Physical Chemistry in Prague on board to study how lipids are transported between cellular organelles. Artificial lipids under the sunbed "We started our project with synthesizing a set of minimally modified lipids that represent the main lipids present in organelle membranes. These modified lipids are essentially the same as their native counterparts, with just a few different atoms that allowed us to track them under the microscope," explains Kristin Böhlig, a Ph.D. student in the Nadler group and chemist who was in charge of creating the modified lipids. The modified lipids mimic natural lipids and are "bifunctional," which means they can be activated by UV light, causing the lipid to bind or crosslink with nearby proteins. The modified lipids were loaded in the membrane of living cells and, over time, transported into the membranes of organelles. The researchers worked with human cells in cell culture, such as bone or intestinal cells, as they are ideal for imaging. "After the treatment with UV light, we were able to monitor the lipids with fluorescence microscopy and capture their location over time. This gave us a comprehensive picture of lipid exchange between cell membrane and organelle membranes," concludes Kristin. In order to understand the microscopy data, the team needed a custom image analysis pipeline. "To address our specific needs, I developed an image analysis pipeline with automated image segmentation assisted by artificial intelligence to quantify the lipid flow through the cellular organelle system," says Juan Iglesias-Artola, who did the image analysis. Speedy lipid transport by proteins By combining the image analysis with mathematical modeling done by Drobot at the HZDR, the research team discovered that between 85% and 95% of the lipid transport between the membranes of cell organelles is organized by carrier proteins that move the lipids, rather than by vesicles. This non-vesicular transport is much more specific with regard to individual lipid species and their sorting to the different organelles in the cell. The researchers also found that the lipid transport by proteins is ten times faster than by vesicles. These results imply that the lipid compositions of organelle membranes are primarily maintained through fast, species-specific, non-vesicular lipid transport. In a parallel set of experiments, the group of Shevchenko at the MPI-CBG used ultra-high-resolution mass spectrometry to see how the different lipids change their structure during the transport from the cell membrane to the organelle membrane. A boost for lipids in cell biology and disease This new approach provides the first-ever quantitative map of how lipids move through the cell to different organelles. The results suggest that non-vesicular lipid transport has a key role in the maintenance of each organelle membrane composition. Honigmann, research group leader at the BIOTEC, says, "Our lipid-imaging technique enables the mechanistic analysis of lipid transport and function directly in cells, which has been impossible before. We think that our work opens the door to a new era of studying the role of lipids within the cell." Imaging of lipids will allow further discoveries and help to reveal the underlying mechanisms in diseases caused by lipid imbalances. The new technique could potentially help to develop new druggable targets and therapeutic approaches for lipid-associated diseases, such as nonalcoholic fatty liver disease. 'We knew that we were onto something big' Nadler, research group leader at MPI-CBG, looks back at the start of the study: "Imaging lipids in cells has always been one of the most challenging aspects of microscopy. Our project was no different. Alf Honigmann and I started discussing about solving the lipid imaging problem as soon as we got hired in close succession at MPI-CBG in 2014/15 and we quickly decided to go for it. "It still took us almost five years from the start of the project to the point in autumn 2019 when the two of us finally produced a sample with a beautiful plasma membrane stain. That's when we knew that we were onto something big. As a reward, certain well-known global events meant we were required to shut down our laboratories a few months later. "In the end, the delay was for the best. Before the revolution in the use of artificial intelligence in image segmentation, we would not have been able to properly quantify the imaging data, so our conclusions would have been much more limited." Researchers still need to determine which lipid-transfer proteins drive the selective transport of different lipid species. They also need to identify the energy sources that power lipid transport and ensure that each organelle keeps its own unique membrane composition.
[2]
Visualizing lipid transport inside living cells with fluorescence microscopy
Max Planck Institute of Molecular Cell Biology and Genetics (MPI-CBG)Aug 29 2025 To the point: New technique to image single lipids: Lipids are notoriously difficult to detect with light microscopy. Using a new chemical labeling strategy, the Dresden team has overcome this limitation, enabling novel insights into where specific lipids are located and how they are transported in cells. Map of lipid flow: The researchers used the new lipid imaging method to answer the long-standing question how cells transport specific lipids to their target organelle membranes. The study revealed that non-vesicular lipid transport by proteins is the primary mechanism that maintains the membrane composition of specific organelles. Understanding the role of lipids in diseases: Lipid imbalances play a role in several metabolic or neurodegenerative diseases. The new lipid-imaging technique will help understand the role of lipid transport in health and disease. The identification of the proteins involved in selective lipid transport can accelerate further discoveries of new drug targets for lipid-associated diseases. Lipid molecules, or fats, are crucial to all forms of life. Cells need lipids to build membranes, separate and organize biochemical reactions, store energy, and transmit information. Every cell can create thousands of different lipids, and when they are out of balance, metabolic and neurodegenerative diseases can arise. It is still not well understood how cells sort different types of lipids between cell organelles to maintain the composition of each membrane. A major reason is that lipids are difficult to study, since microscopy techniques to precisely trace their location inside cells have so far been missing. In a long-standing collaboration André Nadler, a chemical biologist at the Max Planck Institute of Molecular Cell Biology and Genetics (MPI-CBG) in Dresden, Germany teamed up with Alf Honigmann, a bioimaging specialist at Biotechnology Center (BIOTEC) at the TUD Dresden University of Technology, to develop a method that enables visualizing lipids in cells using standard fluorescence microscopy. After the first successful proof of concept, the duo brought mass-spectrometry expert Andrej Shevchenko (MPI-CBG), Björn Drobot at the Helmholtz-Zentrum Dresden-Rossendorf (HZDR), and the group of Martin Hof from the J. Heyrovsky Institute of Physical Chemistry in Prague on board to study how lipids are transported between cellular organelles. Artificial lipids under the sunbed "We started our project with synthesizing a set of minimally modified lipids that represent the main lipids present in organelle membranes. These modified lipids are essentially the same as their native counterparts, with just a few different atoms that allowed us to track them under the microscope," explains Kristin Böhlig, a PhD student in the Nadler group and chemist who was in charge of creating the modified lipids. The modified lipids mimic natural lipids and are "bifunctional," which means they can be activated by UV light, causing the lipid to bind or crosslink with nearby proteins. The modified lipids were loaded in the membrane of living cells and, over time, transported into the membranes of organelles. The researchers worked with human cells in cell culture, such as bone or intestinal cells, as they are ideal for imaging. After the treatment with UV light, we were able to monitor the lipids with fluorescence microscopy and capture their location over time. This gave us a comprehensive picture of lipid exchange between cell membrane and organelle membranes." Kristin Böhlig, PhD student In order to understand the microscopy data, the team needed a custom image analysis pipeline. "To address our specific needs, I developed an image analysis pipeline with automated image segmentation assisted by artificial intelligence to quantify the lipid flow through the cellular organelle system," says Juan Iglesias-Artola, who did the image analysis. Speedy lipid transport by proteins By combining the image analysis with mathematical modeling, done by Björn Drobot at the HZDR, the research team discovered that between 85% and 95% of the lipid transport between the membranes of cell organelles is organized by carrier proteins that move the lipids, rather than by vesicles. This non-vesicular transport is much more specific with regard to individual lipid species and their sorting to the different organelles in the cell. The researchers also found that the lipid transport by proteins is ten times faster than by vesicles. These results imply that the lipid compositions of organelle membranes are primarily maintained through fast, species-specific, non-vesicular lipid transport. In a parallel set of experiments, the group of Andrej Shevchenko at the MPI-CBG used ultra-high-resolution mass spectrometry to see how the different lipids change their structure during the transport from the cell membrane to the organelle membrane. A boost for lipids in cell biology and disease This new approach provides the first-ever quantitative map of how lipids move through the cell to different organelles. The results suggest that non-vesicular lipid transport has a key role in the maintenance of each organelle membrane composition. Alf Honigmann, research group leader at the BIOTEC says, "Our lipid-imaging technique enables the mechanistic analysis of lipid transport and function directly in cells, which has been impossible before. We think that our work opens the door to a new era of studying the role of lipids within the cell." Imaging of lipids will allow further discoveries and help to reveal the underlying mechanisms in diseases caused by lipid imbalances. The new technique could potentially help to develop new druggable targets and therapeutic approaches for lipid-associated diseases, such as nonalcoholic fatty liver disease. "We knew that we were onto something big" André Nadler, research group leader at MPI-CBG, looks back at the start of the study, "Imaging lipids in cells has always been one of the most challenging aspects of microscopy. Our project was no different. Alf Honigmann and I started discussing about solving the lipid imaging problem as soon as we got hired in close succession at MPI-CBG in 2014/15 and we quickly decided to go for it. It still took us almost five years from the start of the project to the point in autumn 2019 when the two of us finally produced a sample with a beautiful plasma membrane stain. That's when we knew that we were onto something big. As a reward, certain well known global events meant we were required to shut down our laboratories a few months later. In the end, the delay was for the best. Before the revolution in the use of artificial intelligence in image segmentation, we would not have been able to properly quantify the imaging data, so our conclusions would have been much more limited." Researchers still need to determine which lipid-transfer proteins drive the selective transport of different lipid species. They also need to identify the energy sources that power lipid transport and ensure that each organelle keeps its own unique membrane composition. Max Planck Institute of Molecular Cell Biology and Genetics (MPI-CBG) Journal reference: Iglesias-Artola, J. M., et al. (2025). Quantitative imaging of lipid transport in mammalian cells. Nature. doi.org/10.1038/s41586-025-09432-x
Share
Share
Copy Link
Researchers develop a novel technique to visualize lipid transport in cells, revealing that proteins, not vesicles, are the primary means of lipid movement between cellular organelles.
Breakthrough in Lipid Imaging
Researchers from the Max Planck Institute of Molecular Cell Biology and Genetics (MPI-CBG) and the Biotechnology Center (BIOTEC) of TU Dresden have developed a groundbreaking method for visualizing lipids in cells using standard fluorescence microscopy. This new technique has enabled them to answer a long-standing question in cell biology: how do cells transport specific lipids to the membranes of their target organelles?
1
The Challenge of Lipid Visualization
Lipids, essential for various cellular functions, have been notoriously difficult to study due to limitations in microscopy techniques. The inability to precisely trace lipid locations inside cells has hindered our understanding of lipid transport and sorting between cell organelles
2
.Innovative Lipid Labeling Strategy
The research team, led by André Nadler and Alf Honigmann, developed a chemical labeling strategy that overcomes previous limitations. They synthesized minimally modified lipids that represent the main lipids present in organelle membranes. These "bifunctional" lipids can be activated by UV light, causing them to bind with nearby proteins
1
.Methodology and Analysis

Source: News-Medical
The modified lipids were loaded into the membranes of living human cells and tracked over time using fluorescence microscopy. To analyze the vast amount of data generated, the team developed a custom image analysis pipeline using artificial intelligence for automated image segmentation
2
.Key Findings

Source: Phys.org
The study revealed that between 85% and 95% of lipid transport between cell organelle membranes is organized by carrier proteins, rather than by vesicles. This non-vesicular transport is not only more specific to individual lipid species but also ten times faster than vesicular transport
1
.Related Stories
Implications for Cell Biology and Disease Research
This new approach provides the first quantitative map of lipid movement through cells to different organelles. The findings suggest that non-vesicular lipid transport plays a key role in maintaining the composition of each organelle membrane
2
.Future Applications
The lipid-imaging technique enables the mechanistic analysis of lipid transport and function directly in cells, opening new avenues for studying the role of lipids within cells. This breakthrough could potentially help in developing new drug targets and therapeutic approaches for lipid-associated diseases, such as nonalcoholic fatty liver disease
1
.Collaborative Effort
The success of this project was the result of a collaborative effort involving experts from various institutions, including mass-spectrometry expert Andrej Shevchenko (MPI-CBG), Björn Drobot at the Helmholtz-Zentrum Dresden-Rossendorf (HZDR), and the group of Martin Hof from the J. Heyrovsky Institute of Physical Chemistry in Prague
2
.This groundbreaking research not only advances our understanding of cellular lipid dynamics but also paves the way for future discoveries in cell biology and potential treatments for lipid-related disorders.
References
Summarized by
Navi
Related Stories
Moscot: AI-Powered Technology Revolutionizes Cell Tracking in Organ Development
23 Jan 2025•Science and Research

SCP-Nano: Revolutionary AI-Powered Technology Visualizes Nanocarriers at Single-Cell Level
15 Jan 2025•Science and Research

AI Model Predicts Protein Location in Human Cells, Advancing Disease Research and Drug Development
16 May 2025•Science and Research
Recent Highlights
1
ByteDance's Seedance 2.0 AI video generator triggers copyright infringement battle with Hollywood
Policy and Regulation

2
Demis Hassabis predicts AGI in 5-8 years, sees new golden era transforming medicine and science
Technology

3
Nvidia and Meta forge massive chip deal as computing power demands reshape AI infrastructure
Technology





